Signal transmission paths
Signal transmission paths
Introduction
Once the signaling molecule (ligand) of one cell binds to the receptor of another, is the signaling process complete?
If we are talking about intracellular receptors that bind their ligand inside the cell and directly activate genes, the answer could be yes. In most cases, however, the answer is no, not at all! In the case of receptors located on the cell membrane, the signal must be transmitted by other molecules within the cell, in a kind of "telephone" game.
The chains of molecules that transmit signals within a cell are known as intracellular signal transduction pathways. Here we will see the general characteristics of intracellular signal transduction pathways, as well as some transmission mechanisms commonly used in these pathways.
Binding initiates a signaling pathway
When a ligand binds to a cell surface receptor, the receptor's intracellular domain (the region inside the cell) changes in some way. It usually takes on a new form that can activate it as an enzyme or allow it to bind to other molecules.
Changes in the receptor set in motion a series of steps in signaling. For example, the receptor can activate another signaling molecule within the cell, which in turn activates its own target. This chain reaction can eventually lead to a change in the characteristics or behavior of the cell, as shown in the illustration below.
Cartoon schematic showing how the components of a hypothetical signaling pathway are activated sequentially. Each one turns on the next to produce a cellular response.
Cartoon schematic showing how the components of a hypothetical signaling pathway are activated sequentially. Each one turns on the next to produce a cellular response.
Because the flow of information is directional, the term upstream is often used to describe the molecules and events that occur first in the chain of transmission, while downstream is used to describe those that come later (in relation to each other) . to a specific molecule of our interest). For example, in the diagram, the receptor is downstream of the ligand but upstream of the proteins in the cytosol. Many signal transduction pathways amplify the initial signal, such that one ligand molecule can cause activation of many downstream target molecules.
The molecules that transmit a signal are usually proteins. However, non-protein molecules such as ions and phospholipids can also play important roles.
Phosphorylation
The illustration above features several balloons (signaling molecules) labeled "on" or "off." What does it actually mean when a globe is on or off? Proteins can be turned on or off in various ways. One of the most common ways to alter the activity of a protein is the addition of a phosphate group to one or more sites on the protein, a process called phosphorylation.
{Diagram of a phosphorylated protein that has a phosphate group attached to a serine, showing the actual chemical structure of the bond.}
Phosphate groups cannot be attached to any part of a protein. They are usually attached to one of three amino acids that have hydroxyl (-OH) groups in their side chains: tyrosine, threonine, and serine. The transfer of a phosphate group is catalyzed by an enzyme called a kinase, and cells have many different kinases that phosphorylate different target molecules.
Phosphorylation often acts as a switch, but its effects vary from protein to protein. Sometimes phosphorylation makes the protein more active (by increasing catalysis or allowing it to bind to another molecule, for example). In other cases, phosphorylation can deactivate the protein or cause it to break down.
[How can a phosphate group do all this?
Ans: How can the addition of a small phosphate group cause such a large effect on the behavior of a protein? The answer lies in basic biochemistry: to add a phosphate group is to attach a large array of negative charges to the surface of the protein.
This negative charge can attract or repel amino acids within the same protein, causing its shape to change. Because a protein's function depends on its structure, changing its shape can alter its ability to function as an enzyme, increasing or decreasing its activity. Alternatively, phosphorylation can provide a docking site for an interaction partner (say one with a bunch of positive charges) or prevent binding with another molecule.
These are just a few examples, but they give an idea of how a phosphate group can directly affect the chemical behavior of a protein.]
In general, phosphorylation is not permanent. Cells have enzymes called phosphatases that return proteins to their unphosphorylated state by removing a phosphate group.
Cartoon diagram showing how a kinase phosphorylates a protein by adding a phosphate from ATP, generating ADP as a byproduct, and how a phosphatase dephosphorylates it, releasing Pi (inorganic phosphate). Both reactions make up a cycle in which the protein alternates between two states.
Example of phosphorylation: the MAPK signaling cascade
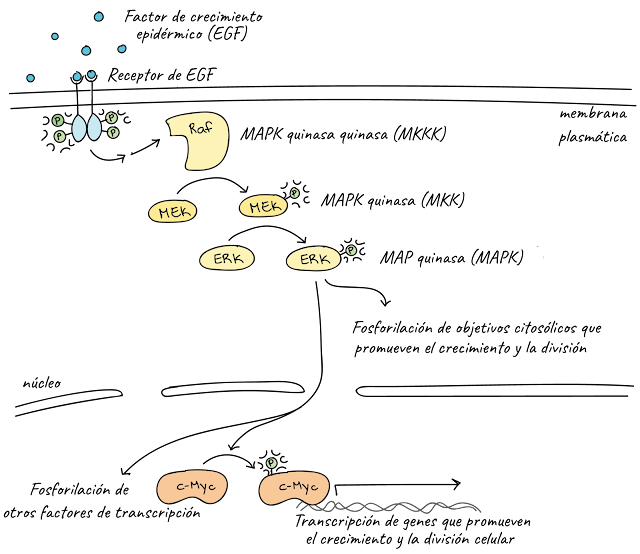
To get a better idea of how phosphorylation works, let's examine a real-world example of a signaling pathway that uses this technique: growth factor signaling. Specifically, we'll look at a part of the epidermal growth factor (EGF) pathway that acts through a series of kinases to produce a cellular response.
This diagram shows part of the epidermal growth factor signaling pathway:
Phosphorylation (labeled P) is important at many steps in this pathway.
- When growth factor ligands bind to their receptors, the receptors form pairs and act as kinases: they bind phosphate groups on the intracellular tails of each other. Read more about it in the receptors and ligands article .
- Activated receptors trigger a series of steps (omitted here because they do not involve phosphorylation) that activate Raf kinase.
- Raf in active form phosphorylates and activates MEK, which in turn phosphorylates and activates ERKs.
- ERKs phosphorylate and activate various target molecules including transcription factors such as c-Myc and cytoplasmic targets. Activated target molecules promote cell growth and division.
Together, Raf, MEK, and the ERKs form a three-tier kinase signaling pathway called the mitogen-activated protein kinase ( MAPK ) cascade. (A mitogen is a signal that causes cells to undergo mitosis , that is, to divide.) Because they play a central role in promoting cell division, the genes that code for the growth factor receptor, Raf and c-Myc, are proto-oncogenes, meaning that overly active forms of these proteins associate with the Cance.
MAP kinase signaling pathways are widespread in biology: they are found in a wide variety of organisms, from humans to yeast to plants. The similarity of MAPK cascades in various organisms suggests that this pathway appeared early in the evolutionary history of life and was already present in a common ancestor of modern plants, animals, and fungi.
Second Messengers
Although proteins are important in signal transduction pathways, other types of molecules may be involved as well. Many pathways involve second messengers, non-protein molecules that pass on the signal initiated by the binding of a ligand (the "first messenger") to its receptor.
Second messengers include ions That 2+; cyclic AMP (cAMP), a derivative of ATP; and inositol phosphate, which is made up of phospholipids.
Calcium Ions
Calcium ions are a widely used type of the second messenger. In most cells, the concentration of calcium ions 2+ in the cytosol is very low, since ion pumps in the plasma membrane continuously work to move them out of the cell. For signaling purposes, 2+ can be stored in compartments such as the endoplasmic reticulum.
In pathways that use calcium ions as second messengers, upstream signaling steps release a ligand that binds to and opens ligand-gated calcium ion channels. These channels open up and allow high levels of 2+ present outside the cell (or within intracellular storage compartments) enter the cytoplasm, raising the concentration of 2+ cytoplasmic
How does it help transmit the signal?
Some proteins in the cell have binding sites for ions. The released ions bind to these proteins, changing their shape (and thus their activity). The proteins present and the response produced is different in different types of cells. For example, signaling by 2+ in the β cells of the pancreas, it produces the release of insulin, while in the muscle cells, the 2+ produces muscle contraction.
AMP cyclic (AMPc)
Another second messenger used in many different cell types is cyclic adenosine monophosphate ( cyclic AMP or cAMP ), a small molecule derived from ATP. In response to the signals, an enzyme called adenylate cyclase converts ATP to cAMP by removing two phosphates from it and attaching the remaining phosphate to the sugar to form a ring.
[See the reaction that converts ATP to cAMP]
Once generated, cAMP can activate an enzyme called protein kinase A ( PKA ), allowing it to phosphorylate its targets and thus transmit the signal. Protein kinase A is found in various types of cells and has different target proteins in each. Thus, the same cAMP second messenger can elicit different responses in different contexts.
Diagram of a pathway that uses cAMP as a second messenger. A ligand binds to a receptor, indirectly leading to the activation of adenylate cyclase, which converts ATP to cAMP. cAMP binds to and activates protein kinase A, allowing PKA to phosphorylate downstream factors to produce a cellular response.
cAMP signaling is turned off by enzymes called phosphodiesterases , which break down the cAMP ring and convert it to adenosine monophosphate (AMP).
Inositol Phosphates
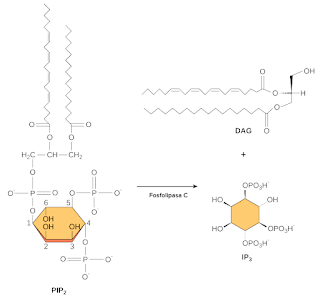
We generally think of plasma membrane phospholipids as structural components of the cell, however, they can also be important participants in signaling. Phospholipids called phosphatidylinositols can be phosphorylated and split in half, releasing two fragments that act as second messengers.
One lipid in this group that is particularly important in signaling is PIP 2. In response to a signal, an enzyme called phospholipase C splits (cuts) thePIP 2 in two fragments, DAG eIP3. Both fragments can act as second messengers.
[See chemical structures and reaction]
DAG remains in the plasma membrane and activates a target molecule called protein kinase C (PKC), allowing it to phosphorylate its own targets in turn. The IP3 diffuses into the cytoplasm and binds to ligand-gated calcium channels in the endoplasmic reticulum, releasing Ca2+ which continues the cascade of signals.
Image of a signaling pathway that uses inositol triphosphate and calcium ions as second messengers. After a ligand binds to a receptor on the membrane, it indirectly activates phospholipase C, which cleaves PIP2 to produce IP3 and DAG. DAG remains in the membrane and activates protein kinase C, which in turn phosphorylates its target molecules. IP3 is released into the cytosol where it binds to a calcium ion channel in the endoplasmic reticulum, causing the channel to open. Calcium ions stored in the ER exit the cytosol and bind to calcium-binding proteins. These, in turn, trigger a cellular response.
And...it's even more complicated than that!
Signaling pathways can quickly become complicated. For example, the full version of the epidermal growth factor signaling pathway we looked at earlier looks like a huge ball of fur and would fill an entire poster if you tried to draw it! You can see for yourself in Sal's video on the MAPK track .
This complexity arises because pathways can, and often do, interact with one another. When the pathways interact, they essentially allow the cell to perform logical operations and "calculate" the best response to multiple sources of information. For example, signals from two different pathways may be required to activate a response, which is similar to the logical "AND" operation. Alternatively, if either way can trigger the same response, it will be like performing the logical "OR" operation.
The diagram to the left: the logical "AND" operator in a cell signaling pathway. In order to be activated and produce a response, an intermediate must be phosphorylated on two different residues, one for each pathway. The response occurs only if the first AND the second pathway are active. Right diagram: the logical "OR" operator in a cell signaling pathway. To activate and produce a response, an intermediate must be phosphorylated at a single residue, and either of two pathways can phosphorylate the same residue. The response occurs if either the first OR the second pathway is active.
Diagram to the left: the logical "AND" operator in a cell signaling pathway. In order to be activated and produce a response, an intermediate must be phosphorylated on two different residues, one for each pathway. The response occurs only if the first AND the second pathway are active.
Right diagram: the logical "OR" operator in a cell signaling pathway. To activate and produce a response, an intermediate must be phosphorylated at a single residue, and either of two pathways can phosphorylate the same residue. The response occurs if either the first OR the second pathway is active.
Another source of complexity in signaling is that the same signaling molecule can produce different results depending on which molecules are present in the cell.
For example, the ligand acetylcholine produces opposite effects in skeletal muscle and cardiac muscle because these cell types produce different types of acetylcholine receptors that trigger different pathways.
Cell type specificity in response to acetylcholine. Left panel: skeletal muscle cell. The acetylcholine molecule binds to a ligand-gated ion channel, causing it to open and allowing positively charged ions into the cell. This generates muscle contraction. Right panel: cardiac muscle cell. The acetylcholine molecule binds to a G protein-coupled receptor, triggering a downstream response that causes inhibition of muscle contraction.
Cell type specificity in response to acetylcholine.
Left panel: skeletal muscle cell. The acetylcholine molecule binds to a ligand-gated ion channel, causing it to open and allowing positively charged ions into the cell. This generates muscle contraction.
Right panel: cardiac muscle cell. The acetylcholine molecule binds to a G protein-coupled receptor, triggering a downstream response that causes inhibition of muscle contraction.
These are just a few examples of the complexity that makes signaling pathways so challenging, yet fascinating, to study. Intercellular signaling pathways, especially that of epidermal growth factor that we discussed earlier, are a focus of study for researchers developing new anticancer drugs^{7,8}









Comments
Post a Comment