Nernst Equation
Brief table of contents
Nernst Equation
The Nernst Equation enables the determination of cell potential under non-standard conditions. It relates the measured cell potential to the reaction quotient and allows the accurate determination of equilibrium constants (including solubility constants).
Introduction
The Nernst Equation is derived from the Gibbs free energy under standard conditions.
Eo=Eoreduction−Eooxidation (1)
ΔGΔG is also related to EE under general conditions (standard or not) via
ΔG=−nFE (2
with
- nn is the number of electrons transferred in the reaction (from balanced reaction),
- FF is the Faraday constant (96,500 C/mol), and
- EE is potential difference.
Under standard conditions, Equation 22 is then
ΔGo=−nFEo. (3
Hence, when EoEo is positive, the reaction is spontaneous and when EoEo is negative, the reaction is non-spontaneous. From thermodynamics, the Gibbs energy change under non-standard conditions can be related to the Gibbs energy change under standard Equations via
ΔG=ΔGo+RTlnQ (4)
Substituting ΔG=−nFEΔG=−nFE
and
ΔGo=−nFEo into Equation 4,
we have:
−nFE=−nFEo+RTlnQ (5)
Divide both sides of the Equation above by −nF−nF, we have
E=Eo−RTnFlnQ (6)
Equation 6 can be rewritten in the form of log10log10:
E=Eo−2.303RTnFlog10Q (7)
At standard temperature T = 298 K, the 2.303RTF2.303RTF term equals 0.0592 V and Equation 7 can be rewritten:
E=Eo−0.0592Vnlog10Q (8)
The Equation above indicates that the electrical potential of a cell depends upon the reaction quotient QQ of the reaction. As the redox reaction proceeds, reactants are consumed, and thus the concentration of reactants decreases. Conversely, the concentration of the product increases due to the increase in products formation. As this happens, cell potential gradually decreases until the reaction is at equilibrium, at which ΔG=0ΔG=0. At equilibrium, the reaction quotient Q=KeqQ=Keq. Also, at equilibrium, ΔG=0ΔG=0 and ΔG=−nFEΔG=−nFE, so E=0E=0.
Therefore, substituting Q=KeqQ=Keq and E=0E=0 into the Nernst Equation, we have:
0=Eo−RTnFlnKeq (9)
At room temperature, Equation 9 simplifies into (notice natural log was converted to log base 10):
0=Eo−0.0592Vnlog10Keq (10)
This can be rearranged into:
logKeq=nEo0.0592V (11)
The Equation above indicates that the equilibrium constant KeqKeq is proportional to the standard potential of the reaction. Specifically, when:
• K>1,Eo>0K>1,Eo>0, reaction favors products formation.
• K<1,Eo<0K<1,Eo<0, reaction favors reactants formation.
This result fits Le Châtlier's Principle, which states that when a system at equilibrium experiences a change, the system will minimize that change by shifting the equilibrium in the opposite direction.
Example 1
This make sense from a Le Châtlier's Principle, since the reaction strongly favors the products over the reactants to result in a large EocellEcello of 1.103 V. Hence, the cell is greatly out of equilibrium under standard conditions. Reactions that are just weakly out of equilibrium will have smaller EocellEcello values.Application of the Nernst equation in problem solving.
When considering the issue of redox reactions, it often becomes necessary to calculate the electromotive force (EMF) and the potentials of individual half-reactions. Reference books usually contain tables of the so-called. standard potentials of certain processes calculated at p=1 atm, T=298K, and activities of the participants equal to 1. However, in real problems, the conditions may differ significantly from those indicated above. How to be in that case? The answer is given by the Nernst equation. In its original form, it looks like this:
E = reduction potential
E^0 = standard potential
R = universal gas constant
T = temperature in kelvin
z = ion charge (moles of electrons)
F = Faraday constant
Q = reaction quotient
As you can see, there are several constants in the equation. Also,
the temperature in the vast majority of cases is 298K. In addition, you
can replace the natural logarithm with a decimal one. This can be done by
multiplying by a conversion factor. If we collect all the constants into a
single factor, then we come to a slightly different, but more familiar form of
the Nernst equation from textbooks:
This version of the equation greatly simplifies life in a number
of cases, for example, when considering pH-dependent processes. Using this
equation, you can carry out calculations under any conditions given in the
problem. Consider typical examples of assignments on this topic.
Example 2:
Calculate the EMF of a galvanic cell composed of copper and zinc
plates immersed in solutions of 0.1M CuSO 4 and
0.01M ZnSO 4 , respectively. The activity coefficients of Cu 2+ and
Zn 2+ ions are
taken equal to unity.
Solution:
First, let's write down the equations of the ongoing processes:
Next, we find the standard process potentials from the table:
If in the conditions of the problem nothing is said about the
activity coefficients of ions, then they can be considered equal to unity, as
in our case. Then the activities of participants in the processes can be
taken equal to their analytical concentrations.
Let us find real potentials taking into account non-standard ion
activities:
Next, it is necessary to compare the obtained values with each
other in order to determine which of the participants in the process is the
oxidizing agent. The potential of copper is greater than that of zinc, so
it will be an oxidizing agent. Then we find the EMF of the system:
Answer: 1.13
V
Example 3:
One of the laboratory methods for obtaining chlorine is the action
of KMnO 4 on concentrated hydrochloric acid. Is it possible to
carry out the process at pH=4?
Solution:
To begin with, we write down the equations of the ongoing
processes.
Answer: the process is not running.
At the end, we present a general algorithm for solving problems
using the Nernst equation:
- 1) Write the
equations of half-reactions corresponding to the process.
- 2) Determine
in which of the equations the concentrations differ from the standard
ones.
- 3) Determine
the number of electrons involved in the process.
- 4) Calculate
the real potentials using the Nernst equation.
- 5) Answer the question of the task.
Potentiometric methods of analysis
Potentiometric methods are based on the measurement
of electromotive forces (EMF):
E = E 1 -E 2
where E is the electromotive force (EMF);
E 1 and E 2 are the potentials of the
electrodes of the circuit under study.
The electrode potential E is related to the
activity and concentration of substances involved in the electrode process by
the Nernst equation:
|
|
(1) |
where E0 is the standard potential of the redox
system;
R is the universal gas constant, equal to 8.312 J/(mol K);
T - absolute temperature, K;
F - Faraday's constant, equal to 96485 C/mol;
n is the number of electrons taking part in the electrode reaction;
a ox , a red are the activities of
the oxidized and reduced forms of the redox system, respectively;
[ox], [red] - their molar concentrations;
[ox], [red] - activity coefficients.
E=E 0 at a ox =
a red = 1, and this refers to a hypothetical standard 1 M
solution, in which the activity coefficient of each solute is 1, and pure
substances are in the most stable physical state at a given temperature and
normal atmospheric pressure.
Substituting T=298.15 and the numerical values
of the constants into the equation, we get for 25 °C
|
|
|
However, the potential of an individual
electrode cannot be determined experimentally. The relative values of
the electrode potential are found by combining this electrode with a standard
hydrogen electrode, which is a generally accepted international
standard. The potential of the hydrogen electrode is assumed to be zero at
all temperatures, so the potential of this electrode is, in essence, the EMF of
the element consisting of this and the standard hydrogen electrode.
Structurally, the standard hydrogen electrode is
a platinized platinum plate, washed by gaseous hydrogen at a pressure of
1.013 . 10 5 Pa (1 atm) and immersed
in an acid solution with an activity of H + ions equal to
one. During the operation of the hydrogen electrode, the reaction proceeds
H2(г) = 2H+ + 2e-
In practical work, instead of a fragile and
often capricious hydrogen electrode, special, more convenient and stable
reference electrodes are used, the potential of which with respect to a
standard hydrogen electrode is precisely known.
Equation (2) can be rewritten
The quantity E 0 ' is called the formal potential. As can be seen, the formal potential characterizes a system in which the concentrations (rather than activities) of all participants are equal to 1.0 mol/l. The formal potential includes activity coefficients, i.e. depends on the ionic strength of the solution. If the activity factor is equal to 1, then E 0 '=E 0 , i.e. the formal potential coincides with the standard one. The accuracy of this approximation is sufficient for many calculations.
The nature of the emergence of the potential is
different. We can distinguish the following three main classes of
potentials, which, of course, do not exhaust the entire variety. This:
1.
Electrode potentials.
2.
Redox potentials.
3.
membrane potentials.
Although the term "electrode
potential" often refers to any potential, regardless of the mechanism of
its occurrence, in a narrower sense, this is the potential directly associated
with the electrode material. For example, zinc electrode:
Zn 2+ + 2e - =
Zn
The activity of the free metal is assumed to be
unity. Electrode potentials differ from redox potentials, for which the
electrode material does not matter, since they are chemically inert with
respect to all substances in solution, and from membrane potentials, for which
the potential difference across the membrane is measured using a pair of others
(in principle, it is possible identical) electrodes.
Potentiometric methods of analysis have been
known since the end of the last century, when Nernst derived (1889) the
well-known equation (1), and Berend reported (1883) the first potentiometric
titration. The intensive development of potentiometry in recent years is
mainly due to the appearance of various types of ion- selective
electrodes, which allow direct determination of the
concentration of many ions in solution, and success in the design and mass
production of devices for potentiometric
measurements.
Potentiometric methods of analysis are divided
into direct potentiometry (ionometry) and potentiometric titration. Direct
potentiometry methods are based on the direct application of the Nernst
equation (1) to find the activity or concentration of an electrode reaction
participant from the experimentally measured EMF of the circuit or the
potential of the corresponding electrode. In potentiometric titration, the
equivalence point is determined by a sharp change (jump) in the potential near
the equivalence point.
The Nernst equation and its various forms
Redox system, written in general form
aOx + ne – DbRed
corresponds to the most general form of the Nernst equation:
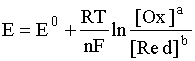 .
.
If we replace the natural logarithm with the decimal one and substitute the corresponding values of the constants in the prelogarithmic factor, then for a temperature of 298 K the equation becomes
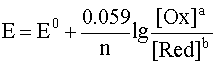 .
.
In the future, we will use the rounded value of the numerical constant in the logarithmic term, which greatly simplifies the calculations without introducing a significant error into their result:
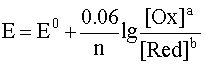 .
.
For example, for the half reaction
Sn 4+ + 2e – D Sn 2+
the Nernst equation has the form:
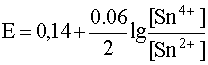 .
.
In various special cases, depending on the nature of the half-reaction, the Nernst equation is written in different ways:
1. Half reactions
aA + bB + ... + ne – D mM + nN + ...
corresponds potential
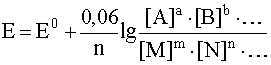 .
.
For example, for a redox system
Cr 2 O 7 2– + 14H + + 6e – D 2Cr 3+ + 7H 2 O
the potential is expressed by the equation
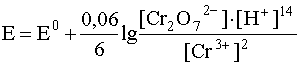 .
.
Let us pay attention to the fact that in sufficiently dilute aqueous solutions, the concentration of water can be considered a constant value, therefore it does not appear in the denominator of the fraction, but is implicitly included in the constant E 0 . This form of the Nernst equation corresponds to the very common and important case when the redox equilibrium proceeds with the participation of the medium.
2. If the redox system includes a poorly soluble substance, then its concentration, being also a constant value, is not included in the logarithmic term of the Nernst equation. So, for the half-reaction
MnO 4 – + 4H + +3e – D MnO 2( Т ) + 2H 2 O
the Nernst equation has the form
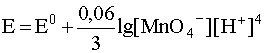 .
.
3. For metal electrodes, that is, for redox systems, which are metal in contact with a solution containing cations of the same metal, for example, for an electrode
Zn 2+ + 2e – D Zn ( T )
the Nernst equation only includes the concentration of metal cations in solution, i.e.
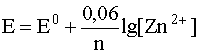 .
.
4. If the redox system includes a gas that is poorly soluble in water (H 2 , O 2 , N 2 , etc.), then the Nernst equation does not include the concentration of this gas, but its partial pressure. For example, for a system
О 2(Г) + 4Н + + 4е – D 2H 2 O
the Nernst equation should be written as follows:
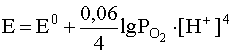 .
.
However, under normal conditions, the partial pressure of the gas is equal to atmospheric, the solution is saturated with this gas, therefore, there is a constant value, and it is included in the constant E 0 , therefore, for this case
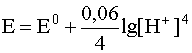










Comments
Post a Comment