Level Beginning To Advance
Introduction to the Nervous System
The world is mostly unknown. This statement immediately
emphasizes the point that we are not conscious of most of the environmental
events that occur around us. The world consists of stimuli of which we may or
may not be aware. These stimuli are pressure variations, chemicals,
electromagnetic radiation, temperature, and even gravity. Figure 1.1
emphasizes this situation. This world of ours contains many events we do not
focus on but also some we simply cannot perceive. We process the sensory
information we interpret automatically each moment. However, we overlook many
interesting aspects of our existence. For example, there are different types of
pain. If you pause and think about it, you can recognize this. Remember the day
you bumped your head. The immediate pain, sharp and crisp, was followed by a
duller but still acutely painful ache and throb. You may even recall being told
to “rub it, it’ll feel better.” The light rubbing usually does reduce the pain,
but what happens if you rub too hard? It does not feel better. Pain is a
confusing sensation when examined closely. Another example is to stare at a
waterfall for a minute and then look at the grass. You would see the grass grow
upward right in front of you. This illusion is the response of an active and
normal visual system.
In this chapter, we examine the human nervous system and
some principles that govern its operation. The nervous system can be understood
more easily by first partitioning it into smaller components. Even when the
nervous system is partitioned, however, it is cumbersome when first
encountered. The goal of this chapter, and the one that follows, is to provide
the knowledge necessary to appreciate the sensory systems discussed in later chapters. This
chapter concludes with a discussion of some nervous system dysfunctions.
The Nervous System
The old adage that says “You are what you eat” can be more correctly stated,
from a neurological perspective, as “You are what your nervous system permits.” This simply means, in an emphatic way, that your thoughts, feelings,
emotions, sensations, desires, dreams, ideas, creative urges, language, and life
itself is under the control of the most complex structure in the world—your
brain. This, of course, does not mean that there are no other physical structures or systems having important roles in your life—for example, digestive
processes, internal organs, glands, and hormones. However, the nervous system is undoubtedly in control. It is no overstatement to say that the function
of 100 to 120 billion neurons composing the nervous system is one of the
most elusive mysteries of science today. The task of understanding the brain
has been difficult but rewarding. The study of the nervous system is one of the
most intellectually stimulating fields of study. Indeed, it is intriguing to realize that the nervous system investigates the nervous system. This is a simple
matter of one brain investigating itself—a unique situation.
The immense magnitude of the nervous system requires, or demands,
that investigators limit themselves to the study of relatively small and restricted features. Even by investigating small regions at a time, however, investigators are continually amazed at the bewildering complexity. The intricacy
occurs in the realms of functions—what the nervous system does, and
structure—how the nervous system is put together. It is our goal to examine
the operation of the nervous system from only one of the multitude of different perspectives, namely, how does the nervous system receive information
from the environment and, once the information is received, how does it process the information? In other words, how do we “sense” the stimuli in our
environment? Because our lives depend on well-functioning sensory systems,
we seek to understand what the nervous system does to provide us with a
“real” world. The real-world environment also includes internal bodily activities such as stomachaches and joint movements. The nervous system monitors react to and interpret the world external to the body while continually
monitoring the shifting environment within.
The nervous system is commonly partitioned into two parts: the peripheral
nervous system (PNS) and the central nervous system (CNS). These two interacting and communicating systems are in actuality a continuous entities.
The peripheral nervous system effectively merges into the central nervous
system so dividing the nervous system into two separate parts is, in fact, an artificial partition. For the sake of description, however, it is a necessity. Furthermore, the central nervous system is usually partitioned into two additional sections: the brain and the spinal cord. The sectioning of the central nervous
system is a useful procedure that is adhered to for our purpose of exposition.
Figure 1.2 shows, diagrammatically, the division of the human nervous system into the peripheral nervous system and the central nervous system. We
discuss each in turn.
The nervous system has two main parts:
- The central nervous system is made up of the brain and spinal cord.
- The peripheral nervous system is made up of nerves that branch off from the spinal cord and extend to all parts of the body.
The Peripheral Nervous System
The peripheral nervous system in Figure 1.2 shows the 31 pairs of spinal
nerves. They are called “spinal” because they carry information to and from
the spinal cord (Heimer, 1983). 12 cranial nerves conduct information to and from the brain more directly; that is, they do not involve
the spinal cord. We discuss the cranial nerves associated with sensory systems
as we examine each sensory modality. We focus here on the spinal and peripheral nerves and their organization.
Before we discuss the plan of the peripheral nervous system, it is important at the outset to briefly examine the idea of a nerve. All nerves are composed of thousands of small strands of fibers called axons. The axon is the
conducting portion of a neuron. The peripheral nervous system and central
nervous system process the neural impulses conducted by each axon. Many of
these axons are individually wrapped with a covering called myelin. The axons are often gathered together to make a nerve. An analogy may be useful.
We can compare a nerve with a telephone cable. A telephone cable (the nerve)
consists of thousands of individually insulated wires (the axons). Each wire
(axon) is capable of carrying a separate message. The insulation around each
wire is the myelin. In addition, the insulation for each wire in the cable is often
color-coded, as is the myelin; it appears white when viewed with a microscope.
The white appearance indicates to investigators that they are viewing a pathway of the nervous system. Neurons themselves appear gray.
The spinal nerves emerge from both sides of the spinal cord in a very specific manner. They emerge from the dorsal and ventral horns. The words dorsal and ventral refer to the back and belly of the spinal cord, respectively. The
ventral portion of the spinal nerve sends information to muscles and glands
and thus has an efferent function. Efferentrefers to the conveying of information away from the central nervous system. The central portion of the nerve is
also referred to as a “motor” nerve because it is often concerned with the
movement of the skeletal muscles. The dorsal portion of the spinal nerve is afferent in nature and carries information toward the central nervous system.
The afferent portion of the spinal nerve provides the sensory information
while the efferent axons allow the central nervous system to send messages to
muscles, internal organs, and glands. Figure 1.3 shows a simplified diagram of
this arrangement.
Some of the spinal nerves are referred to as mixed nerves because they
contain a mix of sensory (afferent) and motor (efferent) fibers. The classical
division of the spinal nerve in two parts, shown in Figure 1.3(b) as the dorsal
and ventral roots, is known as the Bell-Magendie law—the dorsal root is sensory and the ventral root is motor. Recent evidence has shown, however, that some afferent fibers enter the ventral horn, so the Bell-Magendie “law” may
be more of a rule of thumb than a law.
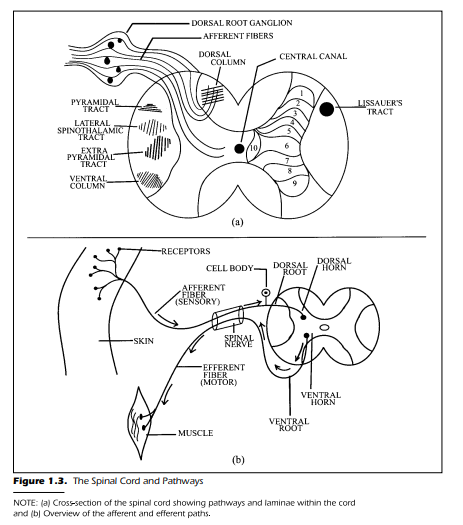
Figure 1.3(a) shows the dorsal root ganglion as an enlargement of the
sensory nerve. The axon fibers that compose the nerve require a cell body or
perikaryon as a means of life support. The cell body is the main protoplasmic
mass of a cell. The cell body produces an internal constituent called axoplasm.
The axoplasm provides the axon with the metabolic means of existence. The
dorsal root ganglion is a gathering together of the cell bodies associated with
each sensory fiber in the spinal nerve. Because the cell bodies are not covered
with myelin, visual inspection yields a gray appearance. Thus, to repeat, one
of the first general rules of the nervous system is that pathways are white and
cell bodies are gray. There is no ganglion for the efferent fibers. The cell bodies
for the efferent fibers are located within the spinal cord itself.
Examination of Figure 1.3(b) shows that the distal end of axons, the end
farthest from the central nervous system, often have special arborizations or
treelike branching near their terminals. In the case of the sensory or afferent
fibers, the arborizations enable the neural element to receive stimulation simultaneously from several sources in the environment. In addition, the axon
often has additional arborizations that permit a single axon to send impulses
to and communicate with many other cells. At the distal end of the neural element, there is often a specialized modification. The modification is a receptor
(discussed in the next chapter). The rule to remember at this point is simple:
No receptor = no sensation.
Peripheral and Spinal Nerves
Until now, we have not differentiated between the spinal nerves and the
peripheral nerves. The situation is, at first glance, somewhat confusing. It can
be readily understood, however, by noting that the spinal nerves come directly from the spinal cord and combine to form a peripheral nerve, see Figure
1.4. As the spinal nerve begins its journey toward the periphery of the body, several plexuses occur. Plexus is Latin for “braid.” This means that the
sensory portions of the spinal nerves are composed of individual fibers that
diverge to different peripheral nerves. Each peripheral nerve, as a result, is
made up of fibers from several spinal nerves. The peripheral nerves then continue to specific areas of the body. It is this very organization of the peripheral
nervous system that causes differences in sensitivity as a function of different
types of physiological insult—that is, an injury or surgical procedure. If a peripheral nerve is severed, the sensations are eliminated from a fixed and relatively small, circumscribed area of the body. Each peripheral nerve serves a restricted portion of the body surface. If, on the other hand, a spinal nerve is
cut there may be very little loss in feeling because fibers from other spinal
nerves innervate the same surface area of the body. Figure 1.4 shows, diagrammatically, how this can occur. If spinal nerve A were severed, there would
be little loss of sensitivity at the body surface labeled 1. This is because the
innervations provided by spinal nerve B overlaps with the body surface previously served by spinal nerve A. In short, the loss of the fibers due to the severing of spinal nerve A is offset by the innervations provided by spinal nerve B.
If, however, you were to cut the peripheral nerve, then all three body surfaces
shown in Figure 1.4 would be devoid of innervations. The entire body area
served by the peripheral nerve would be numb.
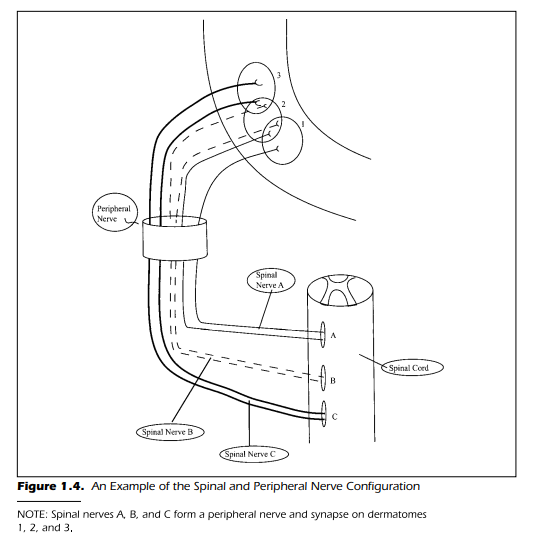
Each circular area of the body surface shown in Figure 1.4 is innervated
by a spinal nerve. Specifically, spinal nerves A, B, and C innervate body surfaces 1, 2, and 3, respectively. The body surface innervated by the dorsal root
of a spinal nerve is called a dermatome. Although each dorsal root (recall that
there are 31 pairs) innervates its own specific dermatome (body surface) the
dermatomes overlap to a large degree.
What this means, most simply, is that the entire surface of the body is partitioned and subdivided into specific areas served by spinal nerves. Figure 1.5
shows the dermatomes of the body associated with the spinal nerves. The dermatomes shown in Figure 1.5 do not overlap as indicated by the previous discussion. Figure 1.5 examines the spinal innervations and the spinal nerves of
the arm. The cervical spinal nerve, labeled C6, innervates the area associated
with the thumb. If you were to cut this spinal nerve, the area associated with
the thumb and associated forearm would be incapable of sending information to the central nervous system. Because the peripheral radial nerve, composed of several spinal nerves, also innervates the thumb, it should be clear
that there still would be some feeling in the thumb. In other words, cutting
spinal nerve C6 does not eliminate all feelings and sensations from the thumb
because there are other spinal nerves serving the thumb area via the radial
nerve. There is a decrease in sensitivity, but not a complete loss of sensation.
The Central Nervous System
As mentioned previously, the central nervous system is composed of two
parts: the spinal cord and the brain. We begin by describing the afferent and
efferent portions of the spinal cord and some of the intricate interactions
within the cord.
We can assume that every pain you perceive depends on information
within your brain. The maxim to remember here is a simple one: No brain, no
pain. There are pathways within the spinal cord that transmit pain information from the extremities of the body. If the spinal cord were severed (a
transaction), you could obtain an idea of how the pathways within the spinal
cord are organized. Figure 1.3(a) shows such a cross-section with the incoming fibers arriving at the dorsal horn. The interior of the cord is shaped like a
butterfly and is gray in appearance. This grayness is a clear cue that one is
viewing millions of unmyelinated cell bodies. The cell bodies, and the
myelinated pathways, have been extensively studied and labeled. The diagram is shown in Figure 1.3(a) briefly introduces the terminology necessary to discuss the conduction and function within the spinal cord. Once we have this
overview, we can continue our discovery within the brain itself.
Spinal Gray Matter
The butterfly shape within the spinal cord is divided into three areas. Two
of the classifications have already been discussed: the dorsal and ventral horns. The third is the intermediate area lying between the two extremes. The
intermediate zone consists of a dense grouping of cells known as interneurons. Interneurons are small neurons that interact with each other within
the layers of the spinal cord. The dorsal horn, intermediate area, and ventral
horn have been further divided into 10 separate layers (see Figure 1.3[a]). There
are five laminae or layers within the dorsal horn, three laminae within the intermediate zone, and two laminae in the ventral horn. There are only about 5
of the 10 that are directly concerned with the transmission and integration of
sensory information. These five are the laminae located in the dorsal horns.
For clarity, the laminae are only shown on the right half of the spinal cord in
Figure 1.3(a). For the time being, we label them from 1 to 5. When we discuss
pain in a later chapter, more details of the function and interaction of these
cellular layers will be apparent. Our goal at this point is to be aware of the fact
that the cellular center of the spinal cord is fairly well defined and has distinctive structural and functional layers.
Spinal Pathways
The pathways that surround the central gray matter conduct information
to the brain—the afferent pathways—and away from the brain—efferent
pathways. Figure 1.3(a) shows, in the white area surrounding the central butterfly, the two major afferent pathways: the dorsal column and the lateral
spinothalamic tract. The afferent pathways are only shown on the left of the
diagram. The pathways in reality, of course, ascend and descend throughout
the area surrounding the butterfly central core. In addition to the afferent
pathways, three major descending paths are coming from higher centers
in the brain: the pyramidal tract, the extrapyramidal tract, and the central
column. At first glance, these paths may appear difficult to remember. However, once you get a feel for how these paths got their names, you can recall
more easily their destination and location. The fibers in the dorsal column
travel throughout the spinal cord and are named according to their dorsal location, near the back. Likewise, the fibers in the lateral spinothalamic tract are
located laterally, to the side, of the spinal cord and conduct information from
the spinal cord to an area within the brain called the thalamus. The lateral
spinothalamic tract has also been called the anterolateral funiculus, the
neospinothalamic tract, paleospinothalamic tract, and the spinoreticular
tract. For our purposes of discussion, we retain the more descriptive nomenclature of the lateral spinothalamic tract.
The pyramidal tract is actually triangular or pyramidal in shape when
viewed in cross section. The extrapyramidal tract is, therefore, just another
pyramidal tract when viewed in cross section. Hence, it is an “extra” pyramidal pathway. The ventral column conducts information down the spinal cord
in the ventral horn,near the belly.Finally,Figure 1.3(a) shows a small pathway
called Lissauer’s tract, named after the individual who first described it.
Many functional aspects of the nervous system got their names from their
discoverers. Lissauer’s tract is relatively short in comparison with the other
pathways. It is located dorsal and lateral to the dorsal horn. The fibers that enter this tract travel a short distance, both up and down, and then reenter the
spinal cord. Thus, information that comes in at one level of the spinal cord
makes contact with other levels of the spinal cord by way of Lissauer’s tract
Although Figure 1.3 is instructive in terms of providing structural labels
and an overall view of the organization of the spinal cord, it is far from complete. If we move on to Figure 1.6, we see a larger perspective of the sensory
path from skin to brain.
Figure 1.6 shows a fiber entering the spinal cord via the dorsal root. Once
the fiber has entered the cord, it joins other fibers already ascending in the
dorsal column. For clarity, the other fibers are not shown. These first-order fibers (first in the sequence) travel upward, enter the brain stem, and make a
connection within an area known as the medulla. Once the first-order fiber
has made this connection the second-order axons leave the medulla and cross
the midline to the opposite side of the body. The ascent then continues toward the thalamus through the pathway called the medial lemniscus (band
or ribbon of fibers). The second-order fibers that enter the thalamus make a
connection with the third and last group of fibers in the sequence. These
third-order fibers then ascend to the cortex and terminate within the primary
somatosensory cortex on the postcentral gyrus of the parietal lobe. Agyrusis
a convolution or bump in contrast with a sulcus that is a groove or fissure. The
entire pathway consists of just three sequences: first-, second-, and third-order neurons, and two connections. An important aspect of the dorsal column pathway is that the fibers enter the spinal cord and ascend ipsilaterally
(on the same side). The fibers do not cross the midline until they have ascended to the brain stem in the medulla. Once they arrive at the brain stem,
they cross the midline and continue their journey to the cortex. The right
hemisphere receives the sensory activity from the left side, and the left hemisphere receives sensory input from the right side.

The course traveled by the fibers within the lateral spinothalamic tract
differs from that in the dorsal column. Figure 1.6 also shows the lateral spinothalamic path. The first-order sensory fibers that make up the lateral
spinothalamic tract connect witht the second-order fibers in the ipsilateral dorsal horn of the spinal cord. Following this first connection, the second-order
fibers immediately cross the midline in the spinal cord to the contralateral
side of the body. The second-order fibers then ascend, via the lateral spinothalamic tract, to the thalamus. Once in the thalamus, the third-order fibers are
contacted to continue the ascent to the postcentral gyrus of the cortex.
Referring back to Figure 1.2, you can see additional information about
the brain structure itself.This view shows the four lobes in the left hemisphere
of the brain: the frontal lobe is behind the forehead, the temporal lobe is on
the side near the temple, the occipital lobe is at the back of the head, and the
parietal lobe is anterior to the occipital lobe and behind the frontal lobe. The
fissure of Rolando, also called the central fissure,separates the frontal and parietal lobe. The central fissure is, from the side view shown in Figure 1.2, located near the central part or center of the brain. The area immediately behind or posterior to the central fissure, postcentral, is the parietal lobe. The
postcentral gyrus is the final destination of afferent sensory information regarding bodily sensations such as touch or pressure on the skin. These portions of the parietal lobe are, therefore, directly associated with the
somesthetic experiences. The particulars of this portion of the cortex are discussed in more detail in a later chapter.
Immediately in front, anterior, of the central fissure is the motor cortex
that initiates the commands for movement. The occipital lobe is the final destination for visual sensations. The temporal lobe has functions for auditory
sensations as well as the capacity to process visual information. In summary,
the pathways for somesthesis terminate at the highest level in the postcentral
gyrus of the parietal lobe. The primary visual processes terminate within the
occipital lobe, and auditory and visual sensations reside within the temporal
lobe. The fissure that runs laterally and divides the temporal lobe from the
frontal and parietal lobes is the fissure of Sylvus. The fissure of Sylvus is also
known as the lateral fissure.
Referring back to Figure 1.2, you can see two functional areas of the brain
known as Broca’s area and Wernicke’s area. These two portions of the brain
are discussed in detail in later chapters. For the moment, it is only necessary to
note that Broca’s area has motor-speech functions and is located in the frontal lobe adjacent to the motor cortex. Broca’s area is directly associated with
phonation, articulation, and facial expression. Speech is directly under the
neural control of a specific area of the brain—namely, Broca’s area.
Wernicke’s area, on the other hand, appears to be responsible for the comprehension and understanding of language. The two areas have connecting pathways. Actually, the complete linguistic dominance of Broca’s and Wernicke’s areas within the left hemisphere is not entirely correct. The language functions are within the left hemisphere for about 90% of right-handed individuals. Left-handed people, however, have their linguistic dominance, speech
production, and comprehension, in the left hemisphere only about 60% of
the time. As usual, the brain and nervous system have shown their typical
complexity.
The description of the nervous system to this point is exceptionally
sketchy. Because there are more than 120 billion cells in the nervous system,
perhaps many more, this is surely an understatement. In addition, there have
been deliberate omissions of specific nuclei, cell groups, and pathways. This
was done to simplify the discussion and still introduce structures, functions,
and nomenclature needed in future chapters. As we proceed, the basic structures of the nervous system are expanded and modified for each sensory system. The meticulous details and the elegant organization of the nervous system are, unfortunately, beyond the scope of a single introductory text.
Nevertheless, the goal is to entice you to examine and wonder about the most
complex and intricate mechanism on earth: your brain.
The Neuron
Thoughts, memories, and all sensations are based on the same brain process. They all operate and depend on the transmission of neural impulses. As
a straightforward analogy, consider the nervous system to be like a giant telephone system. What makes memories, thoughts, vision, speech, hearing, and
pain different is that each system has a different area code and telephone
number. Some of the memories have toll-free “800” numbers. Some of the
numbers are occasionally busy, and some are misdialed. The numbers are all
different but they all work on the same principle—the conductance of electrical impulses along neural pathways. The telephone system has calls going
everywhere simultaneously. Some are routed through the local exchange,
some through an intermediate system, some through communication satellites. The brain uses this same basic idea. The brain has pathways (axons), local exchanges (interneurons), intermediate substations (thalamus), and
higher-level communication satellites (cortex). The points of exchange in the
brain, however, are vastly more complex than any telephone or computer system. Facilitation of transmitted information, inhibition of information, and
modulation or changes in the information occur at billions and billions of
points along the brain’s communication lines. In addition, the neural
paths are monitored by literally millions of other neural paths. Shifts in the information can and do occur because of such monitoring. The bottom line
is that the brain, pathways, and neurons, all operate on the same principle:
electrical-chemical impulses.
A Brief Overview
In this section, we extend our view by focusing on the small neural elements that comprise the central nervous system. These individual parts, the
neurons, provide the foundation for the construction of perceptions. The
uniqueness of the neuron has some similarities with the common digital
computer. Both the computer and the neuron operate on a binary system.
Our brain and the individual cells are, however, several magnitudes more versatile than any computer. No computer, in existence now or even planned for
the future, can ever match the processing abilities of our brain. After describing the distinctive characteristics of neurons, we consider some methods of
recording and measuring their activity.
Neurons, remember, are both the paths to the brain and the centers used
to produce sensory perceptions of the world. Knowledge of their operation is
critical to understanding sensory processes and daily interactions with the
environment. Any error or misfortune in their normal activity affects our
perceptions, thoughts, and memories. It is important that we have this background. All sensory systems are based on these cells operating smoothly.
When neurons fail, we fail.
The Anatomy of a Neuron
The nervous system is based on the electrical-chemical conductance of
impulses over a multitude of paths. The neuron, the basic element of the nervous system, is a physiological structure and a unique entity in itself. Each
neuron or cell is alive and independent, to some degree, of all other cells. It
processes information that impinges on it by integrating (summing) all the
messages it receives and then makes a “decision” whether to send the message
on to other neurons. Even though all the cells operate on the same basic principle, they differ in size, shape, number of arborizations, number of receptive
fibers (dendrites), and chemical messengers (neurotransmitters). In addition, we need to clarify an important element of the analogy. The neuron does
operate on electrical charges and the conduction of impulses; however, the
impulses are dependent on the chemical environment internal and external
to the cell. The role of chemistry is examined more fully in a later section
when we examine the origin of the electrical impulses
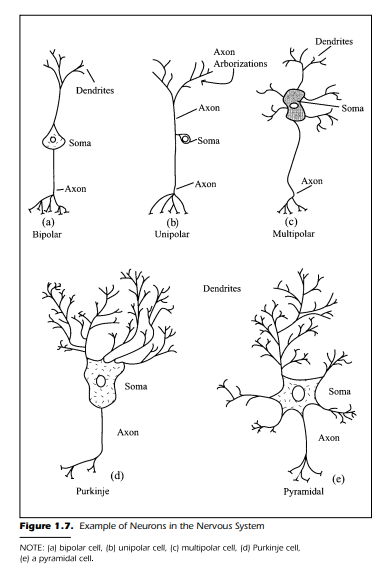
Figure 1.7 is a schematic drawing of neurons found in different parts of
the nervous system. The cells, although they differ radically in shape, have several important features in common. Each neuron generally has four morphological regions:
1. The cell body (also called the soma or perikaryon)
2. Dendrites
3. Axon
4. The terminal end bouton
The end bouton is also referred to as the “presynaptic terminal” at the end of
the axon arborizations. Each part of the neuron has a function of its own. Before we examine the common features, it is useful to examine the neurons
shown in Figure 1.7 more closely.
Figure 1.7(a) shows a bipolar neuron. The name, as you may suspect,
comes from the two fibers that originate from the cell body. One of the fibers
is the dendrite and the other the axon. Based on the classical neuron theory,
the dendrite and axon have two distinctively different functions. The function of the dendrite is to receive information from other cells whereas the
axon conducts the information to the next cell in the sequence. The information flows in a unidirectional path from dendrite to axon. In respect to the bipolar cell shown in Figure 1.7(a), the dendrite receives the information and
the axon sends it on. The bipolar cell can, for example, be found in the visual
system at the back of the eye within the retina. More is said about this type of
neural element when we discuss the visual system.
Figure 1.7(b) shows a neuron that we have discussed, although not specifically by name. This neuron is unipolar and has no dendrites; rather, it has a
single axon emerging from the perikaryon that receives information at one
end and sends it to the other end. This neuron is the common element in the
conductance of information from the skin to the spinal cord. The cell body is
located in the dorsal root ganglion.
A much more common neuron found within the nervous system is the
multipolar cell shown in Figures 1.7(c), 1.7(d), and 1.7(e). Figure 1.7(c)
shows a multipolar cell typical of the interneuron found in the central gray
matter of the spinal cord and brain. The view of the multipolar neuron is
much more complex than the previous two types of cells. There are several
dendrites leaving the cell body. Each dendrite, in turn, has several branches
and collaterals extending from its trunk. The branches also have small dendritic spines to which other neurons make functional contact. The multipolar cell, then, has most of its function devoted to the dendritic reception of inputs
from other cells.
When you consider the fact that a neuron’s soma also receives information in a manner similar to that of the dendrites, the function of the multipolar cell immediately appears to be primarily for reception and integration.
Once the information is integrated, the cell makes a decision. The cell either
forwards the message to the next cell or does not. Figure 1.7(d) and 1.7(e)
show different multipolar neurons. These are the Purkinje cell from the cerebellum and the pyramidal cell from the cortex.
When you think about these cells, particularly the multipolar cells with
their vast number of inputs, the complexity of the brain and nervous system
becomes almost overwhelming. For example, we have already pointed out
that the central nervous system contains an estimated 100 to 120 billion neurons, or more.
When you consider the fact that each multipolar cell probably makes
about 1,000 connections with other cells (the axon has its collaterals and
arborizations) and in turn receives literally thousands of inputs from other
cells, the total number of possible functional connections within a human
brain becomes truly astonishing. Scientists estimate the number of connections is as large as 10,000,000,000,000,000—ten quadrillion, and it is probably an underestimate. This estimate is larger than the estimated number of
stars in our galaxy.
The neurons shown in Figure 1.7 are representative of the diversity found
within the nervous system. Just as in the case of snowflakes, no two neurons
are alike. This single fact makes the nervous system utterly unknowable in
minute detail. Fortunately, the differences among neurons lie primarily in
their morphology, not their basic functional operation. Because of this functional similarity, the telephone cable analogy is quite correct when it comes to
the basic principle that all neurons conduct impulses along predetermined
paths. This principle has allowed scientists to discover the neurological functions that permit an individual to sense the environment, think, have emotions, learn, remember, and be alive.
Our discussion has thus far touched on the function of three of the four
features of a neuron. These are the dendrite, soma, and axon. What remains to
be discussed is the presynaptic terminal or end bouton found at the end of the
axon arborizations. This portion of the neuron is, perhaps, the most important because communication occurs at this point.
The presynaptic terminal, by its very name, suggests that it is only a part
of a more complex structure. This is exactly the case. The functional connection between neurons is called a synapse. The synapse consists of three parts.
The initial part is the structural end bouton found at the end of the axon. The
second part of the synapse is in reality not a physical structure at all; rather, it
is the gap between the axon end terminal and the neural structure on which
the end bouton is functionally attached. The gap is known as the synaptic
cleft. The post side of the synaptic cleft (the end bouton is the presynaptic
side) is referred to as the postsynaptic portion of the functional connection.
Thus, when a synapse is discussed it is in terms of the presynaptic terminal,
the cleft, and the postsynaptic portion of the connection.
You should keep in mind several aspects of the synapse. The vast majority
of synapses occur when an axon connects with a dendrite, axodendritic, or a
soma axosomatic. Often there is a dendritic spine formed on a dendrite for
the synaptic formation. Two other synaptic designations are the axoaxonic,
synapse of one axon on another, and the axoaxonic and axodendritic combination, respectively
A closer view of a representative neuron is provided in Figure 1.8 that
shows a schematic of a multipolar neuron with several synaptic connections
from other neurons. The synapses are axodendritic and axosomatic. Surrounding the single long axon is a myelin sheath. There are separations or interruptions in the myelin called nodes. These nodes, named after the individual who first observed them, are the nodes of Ranvier. The myelin sheath, as
noted previously, is not found on every axon. However, when myelin is present it is the result of a specialized supporting cell. Within the central nervous
system, myelin is formed by a glial cell known as an oligodendrocyte. This cell
wraps itself around the axon in a tight spiral. In the peripheral nervous system, the myelin sheath is the result of a different glial cell called the Schwann
cell. The general effect of having an axon wrapped in the myelin is to improve
the speed of conduction of neural impulses. Unmyelinated fibers conduct
their messages at a much slower rate. The swiftness of conduction in the
myelinated fibers is the result of a process called saltatory conduction. When
an axon is myelinated, the electrical impulses functionally leap from node to
node along the axon.
This “leapfrogging,” from one node of Ranvier to the next, increases the
speed of conduction by a factor of six. At the end of the axon, which in humans may be over a meter in length, are the arborizations and synapses. One
need to exert very little intellectual effort to imagine the length of some of the
axons in giraffes or pachyderms.
The shaded area of Figure 1.8, the point at which the axon leaves the
soma, is known as the axon hillock or the initial segment. This particular section of the neuron plays a key role in determining whether the cell initiates an
impulse within the axon. This section of the neuron is examined more closely
in the following section of this chapter.
The Supporting Glia Cells
The nervous system is not an entity that consists entirely of neurons. In
fact, the nervous system has another group of cells that is 9 to 10 times more
numerous than neurons. (Can you imagine 900 to 1,000 billion more cells in
the brain?) This group of cells is known as glia or sometimes neuroglia.
There are several different subclassifications of glia cells. For our purposes, we need only be concerned with astrocytes, oligodendrocytes,
microglia, and Schwann cells. As noted previously, the oligodendrocytes and
Schwann cells act primarily to provide myelin covering to the axons within
the central nervous system and peripheral nervous system, respectively. The
astrocytes, on the other hand, apparently have nutritive functions (Kimelberg
& Norenberg, 1989). They make contact with neurons while simultaneously
in contact with blood capillaries. In addition, when an injury occurs in the
nervous system both the microglia and the astrocytes become actively engaged in the removal of the debris produced by the trauma and degeneration
of the nerve cells. It is the glia cells, primarily the astrocytes and microglia,
which react to the trauma and energize the recovery process. Unfortunately,
the proliferation of the astrocytes and microglia can also lead to a glial scar.
The glial scar is a possible reason for the lack of axon regeneration within the
central nervous system following an injury. The fact that most central nervous system neurons are no longer capable of cell division accounts for the
lack of new neurons. Some recent evidence suggests that the formation of new
neurons in adult mammals is possible.
A Little History
Let us take a few moments to review the past endeavors of scientists who have given us the current panorama of the synapse and the neuron.
The synapse is perhaps one of the most interesting aspects of the nervous
system.
The history of the discovery and understanding of the synapse covers several decades of intellectual debate and experimentation. There were, before
the advent of the electron microscope and direct observation, differences of
opinion regarding how electrical impulses crossed from one
neuron to the next. According to the classical neural theory espoused earlier,
there is a one-way path from the transmitting axon to a receptive dendrite or
cell body. In the view of one group of investigators, a chemical diffuses across
a synaptic cleft, a gap that was yet to be observed, to accomplish transmission from cell to cell. The chemical hypothesis said that the presynaptic end bouton released a chemical that, when it reached the postsynaptic side, initiated activity in the receiving neuron. Many individuals supported this view. If
there is to be a controversy, however, it is important to have colleagues who
support your theory and a group of colleagues who believe otherwise. Thus,
the opposing camp reported strong and convincing arguments that the synapse is not chemically mediated; rather, they contended that information was
passed from neuron to neuron by the electrical impulse simply being passed
on by a physically present conductor among cells.
The debate continued until the weight of the evidence began to suggest
that the chemical hypothesis was correct. One bit of evidence, for example, to
support the latter position was that the time required for an impulse to cross a
synapse was too long to support the electrical hypothesis. The time required
to cross the synapse was measured to be in the neighborhood of 0.3 to 0.5 milliseconds (0.0003 to .0005 of a second). Although this appears to be a very
short time, it is about the amount of time that is necessary for chemical release, diffusion, and postsynaptic contact. This and other types of evidence continued to act as instigators for scientists to find a chemical that permits the transmission of an impulse from one neuron to the next. The search
has yielded several chemical mediators. These chemical transmitters discussed later, are critically important in the functioning of the nervous system
and the sensory processes we take for granted every day.
The story, however, does not end here. The recognition of chemical transmitter substances and the direct observation of a synaptic gap between the
neurons have secured the chemical mediation hypothesis. This conclusion
does not eliminate the possibility of an electrical conductance by a direct
structural connection between the neurons. The electron microscopist not
only provided evidence to support the chemical mediation but also discovered the evidence for the electrical conductance. There are, in fact, physical
connections called gap junctions between some neurons. These gap junctions provide the necessary conduit for the flow of electrical impulses. The
gap junctions act as a pipeline between neurons. Thus, as is often the case in
science, both of the synaptic hypotheses are correct. The electrical synapse is
in the minority, however. The chemically mediated synapse is far more abundant within the nervous system. The electrical synapse, also called electrotonic transmission, has been known to exist in invertebrates and more recently was found in invertebrates. However, their occurrence is rare in humans.
A final bit of history important in the study of the nervous system is one
that concerns many people. How do scientists determine what neurons, glia,
synapses, and pathways look like and how did they discover the brain’s organization and function?
The answer, which seems so obvious, is more complex than you might
suspect. The obvious conclusion is that they looked with a microscope and
drew pictures of what we saw. However, about 150 years ago anatomy was
done painstakingly by dissection and microscopic study of the parts. It was
not until the 1800s that the appearance of the microscope and methods to
stain tissue with dyes such as silver nitrate were found, and great strides were
made in the morphological study of the nervous system. The microscope
greatly enhanced the anatomist’s ability to trace and follow pathways within
the animals examined. It was well-known at that time that when central nervous system tissues were injured they degenerated. The degeneration of injured tissue provided, then as now, a built-in method of learning about nervous structure and function. When an axon is cut, an axotomy begins to
degenerate distally and proximally from the site of the lesion. The initial degeneration is in the direction of impulse flow, away from the cell body and toward the axon terminal. This is known as anterograde degeneration, also called Wallerian degeneration and orthograde degeneration. Retrograde degeneration refers to the degeneration proximal from the zone of trauma, toward the cell body and dendrite. The loss of myelin, axon, dendrite, and soma
can be mapped by the use of stains and dyes that differentially mark the degenerating parts of the cell. In this way, the origin and destination of the cut fiber can be determined.
Advances in cell study in the 1970s have used enzymes and radioactive
markers to trace the fibers and reveal the morphological details of neurons.
These substances are taken up by the metabolic activity of the neuron. Two
important substances used to delineate the neural elements of a cell are the
enzyme horseradish peroxidase (HRP), and radioactive 14C-deoxyglucose.
The dendrites, soma, and axon are then identified and inspected. Tracing
techniques have yielded the line drawings and photographs published in scientific journals. Knowledge concerning neural structure and pathways continues to grow in quantum leaps.
Single-Cell Recording
You already know by the heading of this section that the study of individual neuronal activity is possible. What is described here is a brief overview of
endeavors that have led to Nobel prizes for scientists, cures for diseases, and
intellectual pursuits for hundreds of people, including you.
When we speak of single-cell recording or unit response, it is important
to keep in mind that there are two basic procedures used to study the activity
of living neurons. Both procedures detect and measure electrical voltage or current and variations in voltage or current. The variations may occur because of normal spontaneous activity or because of deliberate external stimulation under experimenter control. In either case, the objective is to record
neural activity.
The first procedure entails recording electrical activity from outside the
cell. The placement of a small microelectrode, approximately 10 microns in
diameter at the tip, is placed near a living and active cell. This is the extracellular procedure because the electrode does not enter the cell from which it
is recording. When the electrode is placed close to a cell, it is possible to record
the electrical activity of the cell as the response flows by the electrode.
Extracellular recordings do not reveal the small voltage variations within the
neuron; rather, they reveal the relatively large impulses, about 100 millivolts
(mv), that are conducted past the electrode. These impulses are formally
known as the action potentials and are based on the transmission of information from one neuron to another. The action potentials are a large part of the
secret to the nervous system, sensation, movement, thought, memories, and
life itself (Hodgkin, 1964, 1992; Peters, Palay, & Webster, 1991).
The second method of examining the neural activity is intracellular recording. In this procedure, an electrode 1.0 micron or less in diameter is inserted directly into a cell. The electrode may be placed in the soma or within
an axon. When the microelectrode tip is inside the cell, without damaging the
cell, you can examine not only the larger action potentials that are recordable
by the extracellular electrode but also the low-level electrical activity that
leads to action potential generation. These small electrical changes are of different types and are known by various labels, but for the time being we call
these smaller voltage variations, approximately 20 to 25 mv, generator potentials. This label is, in many ways, quite descriptive of these small electrical
changes. These intracellular recordings are more difficult to obtain and reveal
different types of information than does the extracellular procedure. Both
techniques, however, are extremely important in the investigation of the
nervous system and sensory processes.
Electrical Potentials of the Neuron
The stage is set now for the details of neural activity. The morphology has
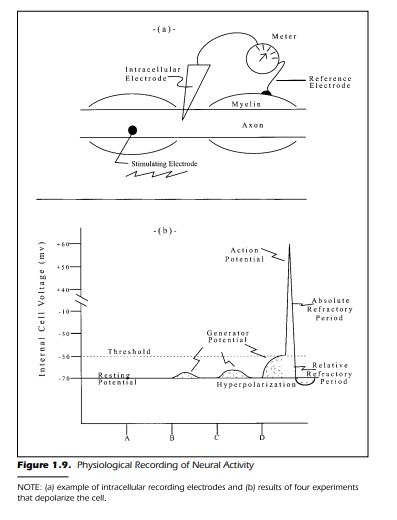
been explained, and the electrodes are waiting for our attention. So let us begin by examining Figure 1.9(a).
This schematic diagram shows an axon with an intracellular electrode
within an axon. The electrode is attached to a meter to indicate changes in
voltage. There must be, as any physics major knows, another electrode placed
somewhere if an electrical circuit is to be completed. This latter electrode, the
reference electrode, is placed outside the cell membrane. This recording system, if it is sensitive enough, should measure the voltage difference across the
cell membrane. Specifically, the two electrodes provide a quantitative difference, in millivolts, between the inside of the cell where the intracellular electrode is placed, and the outside of the cell where the reference electrode is located.
Figure 1.9(b) shows the results of a set of four experiments. Each experiment is an independent study with a different stimulus intensity. The four
stimuli are applied to the neuron at four different times. The stimuli vary in
their strength. Let us assume that the investigator has chosen four values of
increasing magnitude. They are Stimulus A (no stimulus), Stimulus B (weak
intensity),Stimulus C (moderate intensity),and Stimulus D (high intensity).
The experiment is discussed in four stages. Each stage is associated with
one of the four stimulus intensities. We record the voltage across the membrane starting at the point when the stimulus is turned on (a brief electrical
impulse) and stopping when the voltage returns to the starting value. The astute observer notes that there are actually two questions being asked by the investigator in this experiment. First, how does the voltage in millivolts change
as stimulus intensity changes? Second, how does the voltage in millivolts
change as time passes?
Stage I.
The initial recording is done with Stimulus A. In this condition,
we prepare the neural fiber for an electrical shock in the usual manner. However, the intensity of the stimulus is set to 0.0. In this condition, it is assumed
that the voltage measured across the cell membrane is representative of the
cell when it is in an unstimulated or resting state. It is important to emphasize
that the procedures used to record the activity of a neuron with a stimulus intensity equal to 0.0 must be the same procedures as those used when a nonzero stimulus intensity is applied. If valid conclusions are to be drawn from
the experiment, all stimulus conditions must be the same except for stimulus
intensity.
The results of the experiment using Stimulus A is shown in Figure 1.9(b).
The voltage is recorded at a constant –70 mv. The –70 mv is interpreted to
mean that the inside of the cell is negative relative to the outside of the cell.
The neuron, regardless of how you measure it, has a value of –70 mv when it is
inactive and at rest. This voltage is called, not surprisingly, the neuron’s resting potential when it is nonconducting, and unstimulated. This resting potential is the baseline you use when examining the effects of other stimulus
values.
Because the inside of the cell is negative and the outside is positive,the cell
is considered to be polarized in its resting condition. In other words, the cell
has two poles, one negative inside and one positive outside. If you were to depolarize the cell, you need to move the –70 mv potential toward 0.0. If a
stimulus event occurred that caused the voltage difference across the membrane to move toward zero, you could say that the cell was in the process of depolarizing. Any event that depolarizes a cell moves the voltage more positive—
that is, toward 0.0 from a –70 mv. Any event that caused the resting potential
to become more negative could make the cell more polarized. The cell, in this
latter case, is hyperpolarized.
You should keep this terminology firmly in mind as you progress through
the book. A neuron’s momentary state is always considered relative to its
normal resting state. Thus, the terminology of hyperpolarization and depolarization should become second nature as you proceed. If a cell is depolarized, the internal potential is more positive than the resting value; if the cell is
hyperpolarized it has become more negative within the cell.
Stage II.
Stage II of our experiment proceeds exactly as before. However,
in this case a weak stimulus, Stimulus B, is applied. The results are shown in
Figure 1.9(b) as a depolarization of the cell. The application of the stimulus
caused the voltage to move from –70 mv toward 0.0. The magnitude of the
depolarization is small. In a period of approximately 10 milliseconds (msec),
the cell changed from the resting state of –70 mv to –67 mv and back again to
–70 mv. This is the first appearance of the generator potential. Let us try a
more intense stimulus.
Stage III.
The experiment is repeated exactly as before. This time the
stimulus to activate the cell is increased to a moderate value, Stimulus C. Figure 1.9(b) shows the results for this condition. These data are very similar to
those in Stage II with the weak stimulus. The exception is in the magnitude of
the depolarization. The depolarization is 7 mv in Stage III (from –70 mv to
–63 mv). If a conclusion were to be drawn from the data collected thus far it
could run along the following lines: The size of the generator potential is a direct function of the stimulus intensity. The more intense the stimulus, the
greater the depolarization.
Stage IV.
The last stage of the experiment is conducted using Stimulus D,
the most intense of the four levels. The recording begins, as before, when the
stimulus impulse is turned on. The data are collected continuously until the
cell has returned to its resting level of –70 mv.What do the data look like now?
Figure 1.9(b) shows the variation in voltage across the cell membrane as a
function of time after stimulus onset. Figure 1.9(b) shows an initial depolarization when Stimulus D is turned on. It is similar to that seen in Stages II and
III. That is, there is a relatively slow depolarization from –70 mv to –50 mv
during the first few milliseconds following the stimulus onset.This portion of
the data is the generator potential. Following this relatively slow depolarization, a unique feature of the data begins. At –50 mv the internal voltage of the
cell makes a brief and rapid positive-going spike. This rapid depolarization,
and its immediate return to a polarized state, lasts about a millisecond and is,
as previously noted, the action potential. Spike, impulse, or neural impulse are
used interchangeably with action potential. The action potential begins at
–50 mv as indicated by the label threshold. The spike is considered complete
after it has returned to the threshold value. The period of time required for an
action potential to occur,1 msec,is the absolute refractory period.The portion
of the curve immediately following the spike is the relative refractory period.
A portion of this latter interval consists of a period of hyperpolarization.
The experiment is now complete and the results can be interpreted. The
first thing to note is the difference in voltage for the first three stages of the experiment in contrast with the change in voltage in Stage IV with Stimulus D.
In the latter situation, when the most intense stimulus was used, an action potential occurred. The spike was nonexistent in the first three stages of the investigation. If we were to repeat the final stage of the experiment, we could
find the same result every time. Each time Stimulus D, or a stimulus more intense than D, was presented a spike could occur. The interesting aspect of the
replications is that the spike could be initiated at the same voltage level every
time, –50 mv. A fixed amount of depolarization is required to generate a neural impulse. If a spike is to be produced, the cell must be depolarized to a
threshold value. This threshold is –50 mv. If the slow depolarization, the generator potential, does not reach this threshold value, no impulse is produced.
The action potential, then, either occurs or it does not; it is an all-or-none
phenomenon. When the depolarization reaches the threshold value, the impulse is initiated. It completes the cycle from threshold-to-maximum depolarization and back to the threshold in a millisecond.The size of the action potential is always the same for any one neuron.
The conclusions of the experiment may be summarized by noting that
action potentials fire (are generated) on an all-or-none basis and are initiated
when the generator potential reaches the threshold value. The magnitude of
the generator potential is dependent on the stimulus intensity. A weak stimulus produces a small generator potential, also referred to as an electronic depolarization; a stronger stimulus produces a larger generator potential. The
intensity of the stimulus does not affect the size of the action potential. Every
action potential produced by any one neuron is the same magnitude.
Excitation and Inhibition
Thus far, the experiment has worked out well. The results are clear-cut.
The conclusions are straightforward and easily interpreted. The stimulus that
was used to generate the potential variations across the cell membrane was,
however, somewhat loosely defined in the previous paragraphs. The stimuli
were simply defined as electrical shocks with different intensities. The fact of
the matter is, however, that the parameters of the stimulus are critically important in obtaining the observed results. It should come as no surprise that
there are several ways in which the stimulus is applied. We used just one of
them; namely, we applied a brief electrical shock so that the cell depolarized.
The stimuli could be presented in such a way that they increase the negativity
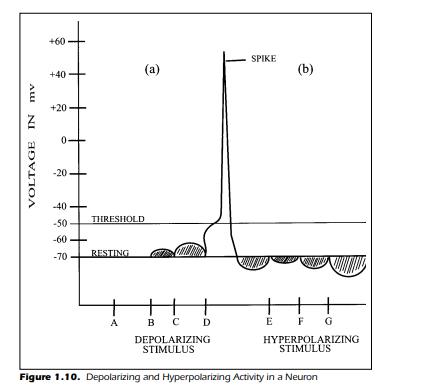
of the cell. If these latter stimuli were used, the results would have been remarkably different. Figure 1.10(a) demonstrates the results of the experiment
we just completed, depolarization and action potential generation, and
shows a new outcome in Figure 1.10(b). This experiment assumes that the
stimulus was reversed in its effect. The internal portion of the cell became
more negative. The stimuli are labeled E, F, and G and represent negative
stimulus intensities in an increasing order of magnitude. The results of such
an experiment are clear: The cell becomes increasingly hyperpolarized and
action potentials never occur.
The results of the entire experiment,including the negative stimulus condition, show that the internal voltage can vary both above and below the resting value of –70 mv. When a stimulus depolarizes the cell, it is possible to generate a spike. When a stimulus causes the cell to become hyperpolarized, there
is no spike generation. It may have occurred to you that if a cell happens to be
hyperpolarized when confronted with a depolarizing stimulus, the cell is less
likely to generate an impulse. This is indeed the case. The further removed
from the critical threshold potential (–50 mv) the cell is, the less likely it is that
the cell becomes depolarized enough to initiate an impulse. If, for example, a
cell is hyperpolarized to –75 mv, the amount of depolarization required to
initiate a spike is 25 mv (from –75 to –50 mv). If the cell is at resting potential,
it requires just 20 mv of depolarization to initiate the spike (–70 to –50 mv). A
cell that is hyperpolarized is in a state of inhibition, and the inhibition must
be overcome if a spike is to be initiated. In contrast with inhibition is the idea
of excitation. As you have already guessed, excitation is associated with the
depolarization of the cell.A stimulus,then,can lead to depolarization (excitation) or hyperpolarization (inhibition).
The ideas of inhibition and excitation are extremely important in understanding the nervous system and sensory processes. The complexity of the
system is almost overwhelming when you consider the fact that each neuron
is at any moment in time in a continuous state of agitation. Furthermore, as
you already know, there are literally thousands of synapses attached to each
neuron. Each one of these synapses is shouting at the top of its “chemical”
voice. Some synapses are excitatory and urgently request that the postsynaptic cell depolarize to immediate activity (excitation). Other synapses
are inhibitory in nature and request an urgent message of inhibition and
Figure 1.10. Depolarizing and Hyperpolarizing Activity in a Neuron
hyperpolarization. You might think that the cacophony of such a situation,
repeated billions of times, would be utter chaos. This, fortunately, is not the
case.Each neuron makes a calm and rational decision.The decision is made at
the initial segment or axon hillock of the neuron (see Figure 1.8) through a
process similar to algebraic summation. That is, excitation (depolarization)
and inhibition (hyperpolarization) are “added up” by the cell with depolarization considered as positive and hyperpolarization as negative. If the result
of the summation is a depolarization to threshold (–50 mv), at the axon hillock, then an action potential is initiated. This summation is a continuous algebraic integration of all synaptic inputs. An action potential is generated
only when the generator potential reaches the threshold value at the initial
segment.
Intensity Coding
It may have occurred to you that the generation of a single action potential, as discussed in the previous experiment, is not sufficient to produce a
sensation, movement, or thought. This is certainly the case. A single impulse
from a single neuron is an insignificant event in the operation of the entire
brain. There are literally billions of action potentials being generated in your
brain and nervous system at this very moment. This occurs even while you
sleep. There is a continuous bombardment of activity from one neuron to the
next, via the synapses, which gives rise to life and active organisms—humans,
cats, dogs, insects, frogs, and so on.
The interesting aspect concerning the brain activity and the sensations
that occur is that everything is done by action potentials. However, the end result of neural activity is clearly not the same. Vision is not the same as touch.
Hearing is certainly different from taste. Yet, all these sensations are based on
the same brain process: the neural impulse and the associated synaptic activity. The reason for the different sensations is, to a large degree, the result of
where the impulses originate and where they are sent. Impulses that originate
from the skin and end up in the somesthetic area of the brain do not produce a visual perception. Impulses from the auditory nerve do not end up in
the visual cortex. Thus, the telephone analogy is accurate in many respects.
This “direct line” concept of neural operation is often referred to as the specificity theory. This theory is encountered repeatedly as we progress through
the book.
Although the specificity theory can help account for intermodality differences, differences between different sensory systems, a question still exits
concerning intramodality differences, differences within the same sensory
system. We all acknowledge that sounds are seldom of equal loudness. The
difference in loudness, an intramodality difference, raises the question:
What is the neural cause for the variation of intramodality sensations?
Neuroscientists, physicians, engineers, and sensory psychologists (among
others) are interested in the processes that cause these various sensations.
The examination of sensory systems has resulted in a variety of procedures and techniques. One fruitful methodological attack was represented by
our previous experiment: The direct neurological or physiological approach.
A second very useful procedure is psychophysics (Kandel, Schwartz, & Jessel,
1995; Posner, 1989). Psychophysics, for the moment, may be briefly introduced by noting that it is a methodological procedure directed toward elucidating the relationship between a stimulus and a sensory response. The stimulus, for example, may be a light or sound of a certain magnitude. The
response may be a simple button press, a verbal response, or an animal’s behavior. Psychophysics finds its primary use in laboratories directed toward
sensory questions using humans and intact organisms. Seldom are invasive
procedures used—for example,no ablation,lesion,axotomy,or single-cell recording. Because you seldom find a human volunteer for a neurological ablation or single-cell recording investigation, the psychophysical procedure is
extensively used with humans. Furthermore, ethical and moral obligations
clearly take precedence with investigations using both human and animal
participants.
Thus, much of the human and animal data come primarily from experiments that use psychophysical procedures. Although there are experiments
that have used human participants with nonpsychophysical procedures,
these latter investigations have occurred under strict ethical conditions, legal
and medical. These latter experiments were accomplished during necessary
brain operations. They are discussed in later chapters. Our aim now is to examine the relationship between the stimulus intensity and the organism’s response, both psychophysically and physiologically.
Physiological Recording
Because we know that sensations are dependent on action potentials,
stimulus intensity must somehow be encoded in the form of neural impulses.
The impulses are dependent on stimulus intensity first because the stimulus
must be intense enough to cause the generator potential to reach threshold
and generate a spike. Thus, one of the goals of investigations in sensory pro-
cesses is to break the neural code and discover how the nervous system generates the multiplicity of sensations from action potentials. A first step in this
enterprise is to examine more thoroughly the relationship between neural
impulses and stimulus intensity.
The procedure we use is the one we are familiar with from the previous
single-cell experiment. The intracellular electrode is implanted within the
cell, and the neutral electrode is placed outside the cell membrane. The independent variable is stimulus intensity. The dependent variable is the variation
in voltage across the cell membrane recorded intracellularly in response to
stimuli of different magnitudes. There is, however, a difference in the duration of the stimulus. In the previous experiment, we had a very brief stimulus
shock. In the present experiment, the stimulus, a depolarizing one, is turned
on and remains on for a longer period. The longer duration of the intracellular recording not only allows us to examine the voltage changes we saw
before but also permits us to count the number of impulses that occur as a
function of the stimulus intensity and duration.
The previous experiment was somewhat artificial because it was assumed
that the stimulus generated only a single action potential. In practice, this is
seldom the case. Any stimulus that evokes a spike, nearly always evokes more
than one. Figure 1.11 shows, diagrammatically, the responses of a neuron to a
stimulus that is long in duration at three different intensities. The stimulus is
denoted by the rectangles below the data in Figure 1.11(a). The most obvious
result is that the number of spikes increases as a function of stimulus intensity. This conclusion is further shown in Figure 1.11(b). The size of the action
potential, as noted previously, does not increase as the stimulus intensity increases; rather, the intensity affects the number or frequency of spikes that occur during the interval. The stronger or more intense the stimulus, the more
neural impulses there are. Part of the neural code, then, is that as the stimulus
in the environment increases in strength, the nervous system increases
the number of action potentials generated. This is the frequency-intensity
principle.
Close inspection of Figure 1.11(a) reveals the manner in which the action
potentials are increased in frequency. The spikes occur more often with more
intense stimuli because each impulse is initiated earlier in the cycle. That is,
after a spike has been generated, the action potential does not completely dissipate or return to resting level before the next impulse is generated. This
means that the interval between spikes decreases as the stimulus intensity increases and, as a result, there are more spikes within the same interval of time.
This results in the increase in the number of action potentials as the intensity
of the stimulus increases. You should note, however, that there is a limit to the
number of action potentials a neuron can produce. At some point, as the
stimulus intensity continues to intensify, the neuron will stop increasing its
spike production. At this point, the neuron has reached its maximum activity
or its saturation point.

Note, once again, that the axis for Figure 1.11(b) is logarithmic. These
data are representative of a power function. There is a linear relationship
when the data are plotted on the log-log scale within the neuron’s dynamic
range. A log-log scale refers to data plotted logarithmically on both the X and
Y axis.
It may appear possible to infer from these data that sensations such as
brightness and loudness are dependent on the frequency-intensity principle.
That is, the sensation depends on the frequency of the neural impulses. For
example, the number of impulses received by the brain within a second indicates the loudness of the sound. This is generally known as a strong inference,
based on reasoned logic and suggestive data. It should be emphasized, however, that the data presented here do not, by themselves, support such an inference. You must be extremely careful in drawing conclusions from data. The
task of finding relationships between sensory experience and neural activity
requires data from several different experiments.
What then can be concluded from the data? What does the coding of intensity to frequency of neural firing show? The acknowledged relationship in
sensory physiology is that a single neuron’s activity is dependent on the stimulus intensity. When several neural fibers increase their activity, there is a perceptual increase in the experienced sensation. So, the frequency-intensity
principle is clearly related to our perceptions.
Action Potentials
This section outlines how neurons process and conduct information. If
environmental events are to be sensed and perceived it is important to understand how a neuron conducts its daily routine. The neuron’s operation includes topics such as passive and active transport, sodium pumps, and action
potentials. The activity that occurs between neurons, at synapses, is the topic
of the next chapter.
The voltage variations across a cell membrane, from a resting potential
near –70 mv to a positive potential near +55 mv has been introduced and discussed. The task now is to explain, as simply as possible, a complex system of
electrical and chemical events. The events provide the basis for the observed
changes across the neural membrane. To be more specific, the question we are
going to address is: What occurs within a cell to maintain a polarized resting
state (–70 mv) and how does depolarization occur during an action potential
(+55 mv)?
The discovery of neural operation has followed a normal course of scientific investigation. Considerable curiosity, perseverance, skill, luck, and intellectual brilliance have led to the present state of knowledge. The breadth of
this knowledge is, of course, still evolving. Experiments are currently underway in laboratories throughout the world that undoubtedly will suggest new
directions and understanding.
Ions and Ionic Flow
The cellular examination of living matter, whether it is nervous tissues or
oak trees, necessitates some knowledge of chemistry and electricity. For our
purposes, the chemical and electrical ideas are relatively simple. For example,
if we put common table salt, NaCl, (called a solute) into a glass of water
(called a solvent) the result is not only a glass of salty tasting water but also the
production of electrically charged substances called ions. The ionic theory
states that, in particular circumstances, the salt molecules dissociate in two
parts called ions. An ion is simply an atom that has gained or lost an extra
electron (or two). When the molecule of salt breaks apart in water, the chloride ion retains an extra orbiting electron that was previously shared. Because
electrons have a negative charge, the chlorine atom takes on a negative charge,
Cl–
. The loss of the electron by the sodium results in a positive ion (Na+
). Furthermore, these positive and negative ions are attracted by electrical potentials with opposite charges because opposite charges attract and like valences
repel. The movement of ions creates ionic flow and is the basis for ion, the
Greek verb that means “to move.”
The internal constituents of a neuron reveals that the cytoplasm or
axoplasm of a neuron contains potassium (K+
), sodium (Na+
), chlorine (Cl–
),
calcium (Ca2+ has lost two electrons), and large amino acids and proteins that
have a negative charge, labeled A–, and called anions.
The concentration of these different ions within the cell, however, is not
equal. There are fewer sodium (Na+
), chlorine (Cl–
) and calcium (Ca2+) ions
inside the cell than there are potassium (K+
) ions. The concentration of K+
within the cell is approximately 20 times higher than outside it. The Na+ distribution, moreover, is almost 9 times more concentrated outside of the cell.
The Cl–
distribution across the cell membrane (inside relative to the outside)
is nearly 5 times greater outside the cell. Finally, the Ca2+ ions have a greater
concentration outside the cell than inside it. These unequal distributions of
ions with their associated electrical charges are shown in Table 1.1.

These
charges and ion distributions are the basis of the negative resting potential
and the action potential. The cell membrane contains “channels” or “pores”
through which ions can flow under certain conditions. This movement of
ions is the aforementioned ionic flow. The flow can be either an influx of ions
into the interior of the cell, or an efflux when ions exit the cell.
When ion channels are open, ionic flow occurs for two reasons—namely,
concentration and electrical gradients. These two gradients form the basis for
passive ion movement.The ions,in turn,determine the resting and action potentials in all neurons.
Concentration and Electrical Gradients
Concentration gradients begin with diffusion, a simple nonmetabolic
process in which there is no expenditure of energy by the cell. Diffusion is the
process by which ions tend to equalize themselves throughout a solution. If,
for example, there were nine ions on the inside of a cell and only one ion on
the outside, this difference could generate a concentration gradient and diffusion could occur provided they could cross the membrane. In an axon, the
uneven distribution of K+ ions on the inside relative to the outside of the cell
leads to diffusion and an ionic flow. The ions attempt to diffuse and equalize
the number of ions on each side of the membrane. When there is a difference
in concentration, an inequality exists. The inequality in ion concentrations
on each side of the membrane results in a concentration gradient. The diffusion of ions from an area of high concentration to an area of low concentration reduces the concentration gradient.
Another reason for ionic flow is electrical. As noted previously, like
charges repel, and unlike charges attract. In terms of ions, a positive area repels positively charged ions such as K+ and Na+
. A negatively charged area attracts the positively charged ions and repels ions like Cl–
. When differences in
TABLE 1.1
Distribution of Ions Across the Cell Membrane
Ion Concentration Inside Concentration
Outside
Ratio
Na+ 50 440 1:9
K+ 400 20 20:1
Cl– 40-150 560 1:6
Ca2+ 0.3 × 10–3 10 —
electrical charge exist between areas, for example, the inside and the outside
of a cell, there is an electrical inequality known as an electrical gradient. For
example, an electrical gradient causes the Na+
ions to flow from an area with a
positive valence or charge to an area with a negative valence. The Na+ influxes
from the outside of the cell to the inside.
The flow of ions may be viewed as analogous to the flow of electrons in a
flashlight. A flashlight battery is a polarized cell, like a neuron. Indeed, the
flashlight battery is called, for example, a “D cell,” an “AA cell,” or a “C cell.”
The voltage, as measured from one pole of the battery to the other, is like the
electrical gradient across a neural membrane. The flow of electrons through a
bulb in the flashlight is analogous to the ionic flow across the cell membrane.
The flow in both cases is due to an electrical gradient.
Passive Transport
It is time to consider a neuron’s charge when the ions are not distributed
equally on both sides of the cell membrane.That is,when the cell is at rest near
–70 mv, what keeps it there and how does it get negatively charged in the first
place?
Similarly, what happens to the ions when an action potential occurs and
the inside of the cell spikes to a value near +55 mv? To answer these questions,
we need to consult Figure 1.12. The sketch is, of course, highly diagrammatic
and inaccurate in terms of how an axon appears. Nevertheless, we can get a
solid feeling for the processes and the general operation of passive transport
by considering the simplified view. Passive transport refers to the movement
of ions without metabolic cell involvement. The neuron does not actively
move the ions across its membrane.
Figure 1.12(a) shows the membrane when there is an equal number of K+
,
Na+
, Cl–
, Ca2+, and anions inside and outside the cell. The voltage across the
membrane is, in this case, balanced because both the concentration and electrical gradients are equal. If we were to arbitrarily remove the K+
ions from the
outside, as shown in Figure 1.12(b), an imbalance is apparent. The inside of
the cell would become positive due to the abundance of the K+
.
In addition,
there would be both a concentration gradient and an electrical gradient
across the membrane. These gradients tend to draw out the K+ from the cell
passively. This is shown by the arrows and the notation Ge and Gc in Figure
1.12(b). In other words, the concentration gradient, Gc, causes the K+ ions to
diffuse out through the membrane and the electrical gradient, Ge, repels the
K+ toward the outside. If nothing occurred to change this situation, the K+
ions eventually distribute themselves equally on each side of the membrane.
The cell is then similar to the one shown in Figure 1.12(a).
If the Na+
, Cl–
, Ca2+, and anion concentrations were distributed across the
membrane as shown in Figure 1.12(c), the situation changes.If the Na+
, Cl–
, Ca2+, and anion concentrat In this case, the
Figure 1.12. Ion Distribution Within an Axon and the Concept of Passive
Conduction
distribution of ions reflects a more realistic situation. It is based on the data
given in Table 1.1.
So far in our discussion, we have assumed that the membrane is impervious to Na+
, Cl–
, Ca2+, and anions and only K+
could pass through. In this situation the inside of the cell is, as noted in Figure 1.12(c), slightly negative relative to the outside. This can be verified in the example by counting the
positive and negative charges on each side of the membrane. The negativity
and the high concentration of K+
inside the cell produce an interesting milieu.
The K+ is, as expected, drawn out of the cell because of the concentration gradient, labeled Gc in Figure 1.12(c). As each K+
ion leaves the cell, due to the diffusion, it takes with it a positive charge. The more the efflux of the K+ ions
continues, due to the concentration gradient “drawing” them out, the more
electrically negative the inside of the cell becomes. Eventually there is an electrical gradient set up across the membrane that equals the concentration gradient, but in the opposite direction. The electrical gradient is labeled Ge in
Figure 1.12(c). The negative valence within the cell draws the K+ ions into the
cell because the K+
ions are positive; simultaneously, the concentration gradient forces the K+ to leave the cell. The result is a stalemate. The concentration
gradient draws K+ out, but only to a certain point. The point of equilibrium is
reached when the K+ no longer diffuses outward because the negativity
within the cell acts as a magnet to draw them back in. When a balance is
reached between the concentration gradient and the electrical gradient, the
voltage drop across the membrane is –75 mv, only 5 mv away from the neural
resting potential.
Figure 1.12(d) shows the situation when the cell is near the resting potential. Ignoring the other ions for a moment, it is clear that the resting potential
of –70 mv is primarily the result of a balance of two forces: the concentration
gradient drawing the K+ out and the electrical gradient, which draws the K+
back in. The K+ ionic flow yields a negative interior of –75 mv, a value very
close to the resting potential. An important point concerning the K+ flow is
that the potential of –75 mv is done without any active help from the cell itself.
The cell is, in this sense, passive. As noted earlier, the process of ionic flow
without metabolic cell activity defines passive transport. Before we consider
the flow of ions during an action potential, it is necessary to digress slightly
and consider an important mechanism known as the sodium-potassium
pump. Once this phenomenon is understood, we can more clearly understand the ionic flow that produces the maintenance of the –70 mv resting
potential and the observed difference of 5 mv.
The Sodium-Potassium Pump
The situations depicted in Figure 1.12 are somewhat artificial. When a
more realistic situation is examined in which the concentrations of Na+
, Ca2+,
and Cl–
are higher on the outside of the cell than within, it becomes apparent
that there is a strong tendency for the Na+ and Ca2+ to attempt to cross the
membrane and enter the cell.The high concentration of Cl–
also sets up a concentration gradient for Cl– to enter the cell. However, this Cl– concentration
gradient is offset by the opposite force of the electrical gradient; Cl–
is driven
out of the cell due to the high negative charge in the cell (due to the A–). We
focus, for the moment, on the Na+ and Ca2+ ions.
Returning to Figure 1.12(d), we see the large number of Na+
and Ca2+ ions
concentrated outside the cell, and the interior of the cell has a negative resting
potential. Both the concentration and electrical gradients for the Na+ and Ca2+
are producing an influx force to move the Na+ and Ca2+ inward. The electrical
gradient produces an influx gradient because the negative resting potential
within the cell attracts the positive sodium and calcium ions. The concentration gradient is an influx force because of the difference in ions inside and
outside the cell. At this point it is time to acknowledge that the cell membrane
is not completely impervious to Na+ and Ca2+. The strong influx gradients
force a leakage of Na+ and Ca2+ through the membrane. The consequence of
the leakage is a decrease in the negativity of the cell interior. The continual
leakage of positive ions into the cell makes the interior of the cell more positive and slowly depolarizes it. Furthermore, as the inside of the cell starts to
become positively charged, the K+ tend to efflux. The K+ leaves because like
charges repel and because there is a concentration gradient attracting the K+
ions outward.
The manner in which the leakage is counteracted
In summary, there is a constant influx of Na+ and Ca2+ ions into the cell
and an efflux of K+
. This constant leakage of ions moves the electrical potential of the cell interior from negative toward positive. If this leakage continues
unabated, the negative resting potential dissipates and eventually becomes
zero. The polarization is removed and the cell “runs down” analogous to a
flashlight that has been turned on all night. If nothing is done by the cell to recharge and prevent the Na+
and Ca2+ ions from leaking in and the K+
ions from
leaving, the negative potential within the cell continues to decrease and eventually becomes zero just as is shown in Figure 1.12(a).
The manner in which the leakage is counteracted entails the active participation of the neuron.The cell transports the Na+
ions back to the outside of the
cell while moving the K+
back inside. This involvement of the cell in the transport of Na+ and K+ is called active transport. To be more specific, the active
transport of Na+
from the inside of the cell to the outside and the active transport of K+ into the cell is accomplished by a metabolic process known as the
sodium-potassium pump. The sodium-potassium pump not only removes
the Na+
from the inside of the cell but simultaneously ensures that the concentration of K+
is maintained at the appropriate level by pumping K+
into the cell.
The sodium-potassium pump does not exchange ions equally, however. The
ratio of exchange is near 3:2; three ions of Na+ are removed for each insertion
of two K+
. This inequality, however, causes no problem because the leakage of
Na+ and the outflow of K+ is at the same rate. Three Na+ ions leak in for every
two K+ ions that leave. Thus, the exchange ratio of the sodium-potassium
pump matches the leakage. The sodium-potassium pump is shown diagrammatically in Figure 1.13. The figure shows the clockwise movement of the
pump as it removes Na+ from the cell and brings K+ into the cell.
Calcium Removal
You have probably noted by now that the active transport system of the
sodium-potassium pump has not handled the infusion of the Ca2+ ions. The
removal of the Ca2+ is done by a different method. Briefly, the Ca2+ removal is a
by-product of the influx of the Na+
. That is, as the Na+ leaks into the cell there
is an automatic and continual removal of Ca2+. An analogy may help to simplify the concept. The in-pouring of the Na+
ions may be viewed as a continuous stream of water that turns a waterwheel. The waterwheel is mechanically
coupled to a large millstone in a flour mill.

As the waterwheel is turned, the
millstone grinds the grain to flour. In this analogy, the Na+ ions represent the
water that turns the waterwheel. The mechanical linkage to the millstone is
analogous to an automatic linkage in the cell membrane. The influx of Na+
ions is the “power” that “mechanically” removes the Ca2+ ions. There is no
special active transport system for the Ca2+ removal. Figure 1.13 shows a situation in which the Na+ and Ca2+ leak into the cell and the sodium-potassium
pump transports the Na+ back out. The sodium-potassium pump also replaces the K+
that has “escaped” to the outside.The mechanical removal of the
Ca2+ occurs when the Na+
leaks into the cell.
Chlorine Distribution
The concentration of Cl–
is predominantly outside the cell relative to the
inside. This, at first glance, tends to cause some concern about their effect on
the potential of the cell. Surprisingly, the effect of Cl–
is relatively small. The
primary reason for such a state of affairs is that the membrane of the cell is
“open” to the free movement of the Cl–
ions. This freedom of movement allows the Cl– ions to arrange themselves in such a manner that they balance,
and the concentration and electrical gradients are equalized at the value of the
resting potential. Said differently, the sodium-potassium pump maintains a
fixed ionic concentration for Na+ and K+ but the Cl–
ions are free to “settle” at
an equilibrium across the membrane that is at the value set by the sodiumpotassium pump.
The 5 mv Difference
To visualize why the resting potential of the cell is –70 mv rather than
–75 mv requires that we acknowledge the actual permeability of the cell
membrane and the activity of the sodium-potassium pump. Let us review the
situation briefly.
Conductance and the Action Potential
When the membrane is permeable only to K+
, then the potential across
the membrane is –75 mv. The assumption is that the membrane is permeable
only to K+
. The assumption is incorrect. There is a tendency for Na+ to leak
into the cell because of the concentration and electrical gradients. Like most
biological systems, the neural membrane is imperfect, it leaks. Consequently,
the membrane is not likely to remain at –75 mv. The influx of Na+ and the
leakage of K+ across the membrane results in a decrease in the internal potential of the cell from the –75 mv. The sodium-potassium pump, of course, operates to offset the leakage of Na+
and K+
, but the pump can only do so if it has
been activated. When the sodium-potassium pump begins operation, it
maintains the membrane potential at a relatively stable value. For our purposes, we can assume that the sodium-potassium pump begins once the cell
has been depolarized from –75 mv to –70 mv. In other words, the sodiumpotassium pump “kicks in” when the potential of the cell reaches –70 mv due
to the Na+
and K+
leakage. As an analogy, ideally, a ship is watertight. In reality,
however, ships do leak. A slight leakage is acceptable if it is maintained at a low
level. If the leakage becomes too great, however, it is important to eliminate it.
The water pumps (bilge pumps) are activated to do the job. In a very leaky
boat the bilge pumps are turned on when the water inside the boat reaches the
maximum allowable limit. Once the pumps are active, the water level is maintained at a constant level. In the analogy, the sodium-potassium pump is activated when enough Na+
and K+
ions have leaked in to increase the internal potential from –75 mv to –70 mv. The sodium-potassium pump maintains a
constant resting state by pumping the leaking Na+
out and returning the K+
to
the inside. The sodium-potassium pump is nearly always active. The only
time the active transport system is not working is during the brief durations
when action potentials are being produced. The generation of the action potential is the next discussion.
Conductance and the Action Potential
One of the important aspects of the neural operation has been alluded to
but not addressed specifically. It should be apparent that ionic movements
across the membrane determine both the resting potential and the action potential. However, what has not been stated is that the membrane of the axon
itself is sensitive to the voltage differential across it. The axon membrane is
potential sensitive.This means that the “pores” or gates that allow the differ-
ent ions to pass through the membrane are opened or closed as a function of
the potential across the membrane.
Figure 1.14 shows a hypothetical view During the resting state, the potential-sensitive gates of the membrane
are open only to the flow of K+
ions (ignoring leakage). The onset of an action
potential begins when a graded potential at the axon hillock reaches the
threshold value of –50 mv. At threshold, the potential-sensitive Na+ gates
open wide to allow a rapid influx of Na+
into the cell. The initial increase in internal potential closes the potential-sensitive K+
gates. The rapid influx of Na+
into the cell continues until the depolarization reaches the +55 mv peak spike
value. The consequent rapid increase in potential has an almost immediate
second effect on the potential-sensitive gates for Na+ ions. At the peak of the
spike (+55 mv), the gates close to the passage of Na+
. An instant later, the gates
open for the efflux of K+ ions from the cell. The efflux of K+
returns the cell to
near resting potential. Said differently, the K+
gates are open and the Na+
gates
are closed when the cell is at rest (–70 mv). When the neuron is depolarized
and reaches threshold, because of the graded potential at the axon hillock, the
Na+ gates open and the K+ gates almost immediately close. This sequence is
then reversed when the internal potential reaches +55 mv. At the peak of the
spike, the Na+
gates close and the K+
gates reopen. The recovery portion of the
action potential that follows the maximum spike potential (+55 mv) is due to
the efflux of the K+
.
Figure 1.14 shows a hypothetical view of the potential-sensitive gates
during rest (Point 1), during a generator potential (Point 2), during the initiation of an action potential (threshold at Point 3), during the action potential
(Point 4), and during the recovery or decline of the action potential (Point 5).
Although the gates are shown diagrammatically opening and closing in unison, this simultaneity does not actually occur. It is just difficult to draw gates
opening and closing with microsecond offsets. The insert at the bottom of
Figure 1.14 indicates the electrical activity at the various points. The result is,
of course, an action potential. The hyperpolarization is shown at Point 6. This
hyperpolarization occurs where the internal potential becomes more negative than the resting potential. This is due to the “overshoot” of K+ ion efflux.
Point 7 reflects a state of rest identical to that at Point 1.
The conductance of an action potential is a relatively simple concept. It is
like the burning of a fuse. Once a fuse has been lit, the heat of the ignited portion raises the temperature of the area adjacent to it. When the powder in the
zone next to the flame reaches its flash point, it too bursts in flame. The flame
at this new point then raises the temperature of the powder next to it, and this
powder now ignites. The flame, therefore, moves steadily down the fuse burn-
ing the powder as it goes. The activity of the neural impulse is similar. During
the occurrence of a spike, the influx of Na+ causes a voltage change across the
cell membrane as it flows into the cell. The voltage change affects the membrane permeability to Na+ in the neighboring area and Na+ begins to enter.
Figure 1.14. Diagrammatic View of Na+
and K+
Gates and the Movement of
Ions During the Generation of an Action Potential
When the threshold value of –50 mv is reached the Na+ gates open wide, and
the spike is conducted to the neighboring area of the membrane. In sum, the
influx of Na+
causes the gates for Na+
to open in the area of the axon next to the
spike. The influx of Na+
is followed by an efflux of K+
. The exchange of ions
flows down the membrane in a manner analogous to that of the fuse.

It is interesting to note another analogy of membrane conductance and
fuses—namely, conductance time. The speed at which the flame moves down
the fuse is dependent, to some degree, on the actual size of the fuse. The larger
the fuse, the faster it conducts the flame to the dynamite. In neural conduction, the size of the axon is also a factor in the speed of conduction. The larger
the axon diameter, the faster it conducts.
There is, finally, one comment necessary to complete the story. Some
neurons have myelination and nodes of Ranvier. The flow of action potentials
in myelinated neurons is increased in speed because of the special mechanism
of saltatory conduction. The myelin acts as a cover that is resistant to the passage of ions through the membrane. Thus, for the neuron to conduct, the action potential must leap from node to node. The speed of conduction is increased when a neuron has myelin. Finally, the size of the neural impulse
(spike) is nondecremental.This simply means that the magnitude of the spike
does not decrease as it is conducted down the axon. In the present example,
the “height” of the spike is maintained at +55 mv from beginning to end.
Dysfunctional
The nervous system is a very delicate and tender structure. As we progress
through the book, selected diseases and accidents are discussed relative to a
normal functioning system. The common everyday experiences and news reports, unfortunately, cover a large range of neurological problems. It is not
the goal of this section to examine the vast array of situations and conditions
that can occur. Rather, these sections of the book, found in nearly every chapter, are directed toward a brief introduction and excursion into some commonly known, and some not so commonly known, dysfunctions (Brodal,
1981).
A serious neurogenic problem is a cerebrovascular accident (CVA),
commonly known as a stroke. The irretrievable loss of neural tissue because
of a stroke is one of the most serious problems an individual can encounter.
The brain, which makes up approximately 2% of the total body weight, requires 17% of all the cardiac output and uses 20% of the oxygen consumption. Nearly 2 million U.S. citizens suffer neurological impairment because of
cardiovascular disease alone.The brain is quite susceptible to interruptions in
the blood and oxygen supply.
A CVA refers to neurological symptoms and signs that occur because of
diseases from blood vessels that serve the brain. The CVA can occur because
the vessels become closed (occlusive CVA), or because the vessels burst (hemorrhagic CVA). In either case, the effect on brain cells can be life threatening
and traumatic. The occlusive CVA is thought generally to be due to atherosclerosis (clogging of the arteries due to fatty deposits) and thrombosis
(blood clots). The hemorrhagic CVA is due to genetic aneurysm, weaknesses
in the vessel wall, or hypertension (high blood pressure). Often when a hemorrhagic CVA occurs there is a loss in consciousness, possibly due to changes
in the intracranial pressures.
A loss of neural cells within the precentral gyrusThe symptoms of a CVA vary according to the region of the brain in
which it occurs. The symptoms presented by the patient provide clues for diagnosing the location of the CVA within the brain. For example, if the loss of
neural cells occurs in the right parietal lobe, there is often a loss of perceptual
abilities with disturbances in the ability to accomplish spatial tasks such as
copying maps, pictures, or diagrams. Occasionally there is a severe difficulty
in finding your way around in the environment, called topographagnosia.
You should keep in mind that the functions affected seldom are controlled by
a single region of the brain. The specificity you find for particular functions
refers to certain regions of the brain that are more concerned with one set of
functions than others. Most functions require integrated actions from several
regions of the cortex as well as lower centers within the brain. Broca’s and
Wernicke’s areas (see Figure 1.2) are examples of specificity, yet it is clear that
without the input from the auditory sensory system, the areas are restricted in
their function. It should be clear at this point that if a CVA were to occur in the
area that produces speech, Broca’s area, the individual’s speech is drastically
affected. A similar CVA within Wernicke’s area results in the loss of the ability
to understand verbal communication.
A loss of neural cells within the precentral gyrus, just in front of the central fissure in the frontal lobe, results in the loss of voluntary movement. A
stroke within the motor area of the left hemisphere, for example, results in
the loss of movement in the right half of the body.A lesion or a loss of cells due
to CVA, or a heavy hit to the head by a boxer, in the posterior parietal cortex usually leave deficits in learning tasks associated with somesthesis. For
example, a CVA in the left hemisphere often shows verbal and language deficits, agnosia or the inability to perceive even though the sensory channel is
functional.
Loss of neural cells in the right hemisphere can result in striking effects.
There sometimes is a complete lack of appreciation for all sensory inputs
from the contralateral side of the body and from the opposite portions of the
environment. Patients often fail to dress, wash, and undress the side of the
body that is contralateral to the neural loss. Such a behavior is called the neglect syndrome.
A final example of a CVA within the temporal lobe is that the loss of neural cells, whether they are few or many, is a serious event. The loss of central
neural cells is permanent. The peripheral nervous system, on the other hand,
has regenerative capabilities. Peripheral nerves do regenerate.
A CVA within the superior temporal lobe in the right hemisphere usually
leads to deficits in auditory pattern recognition. Should the stroke occur
within the medial or inferior portion of the right temporal lobe the result is a
visual learning deficit for patterns. The general outcome of temporal lobe
losses is that loss in the left lobe eliminates the ability to process verbal material and loss in the right hemisphere eliminates the ability to process sensory
pattern information.
A short comment or two needs to be inserted here regarding the regeneration or replacement of central nervous system cells. The first statement has
to do with the “tenderness” of neural lifetimes. You are born with the maximum number of neurons you will ever have. The only sure thing in your life is
that you continue to lose neural cells through the normal attrition of everyday living.
Second, a question is probably hanging around at this point concerning
how it is that people can recover after a traumatic insult to the central nervous
system. Not many years ago very little was known about how such “repairs”
came about. This does not mean that we now fully understand how the brain
comes to take up the slack of injured and destroyed neurons. What is recognized, however, is that the brain has a remarkable ability to take over functions of the neural cells that are destroyed. Not all functions, of course, are recoverable. It depends on the extent of the injury or stroke. The brain has
shown a high degree of what is called neural plasticity—that is, the ability to
do some considerable reorganizing or remodeling, both structurally and
functionally.Because the brain may not produce new nerve cells after a stroke,
lesion, or other disease, it is necessary to examine other mechanisms of
recovery. It has long been recognized that the initial improvement, over the
first few days or weeks,is likely due to the decrease in the edema (i.e.,retention
of fluid or swelling of the brain), resorption of the blood, and the recovery of
the injured cells. The continual recovery of a patient over an indeterminate
period requires another explanation.
Without doubt, many people have viewed the recovery following severe
brain damage with amazement. The reasons for partial or total recovery of literally millions of damaged brain cells require some explanation. A complete
answer, however, is not yet available, although research on brain functions
and structures has suggested three possibilities to account for recovery.
One idea is that the neural elements that have not been affected by the insult or damage caused by the CVA take up the slack by sprouting new
collaterals. The new collaterals make both structural and functional connections with neural elements that, before the damage, were in contact with the
destroyed cells. That is, there may be a new pathway provided and thus the
functions may be reintroduced.
A second alternative for the recovery is that of “unmasking.” This idea
simply means that the death of brain cells can lead to the revelation of unknown or seldom used pathways that accomplish the same function as the
cells that were destroyed by the CVA. Pathways and cells, which were functional before the death of the cells, come to be primary rather than secondary
when the initial primary group of cells is destroyed.
The third possibility, one that surely is involved to some extent, is that of
learning. If learning occurs in normal individuals, as we certainly know it
does, it requires that the brain be capable of modifying its structure and circuitry in some manner. It makes sense to assume that the improvement that
comes about is the result of such learning processes even if it is in a defective
or damaged nervous system. What the exact rewiring is and how plasticity is
accomplished still remains to be determined. There are, certainly, amazing
things on the horizon when it comes to the brain.
The problems that can occur with the neuron are mostly degenerative
diseases and viral invasions. One of the more unfortunate, and fatal, of such
degenerative diseases is that commonly referred to as Lou Gehrig’s disease or
amyotrophic lateral sclerosis (ALS). This disease progressively attacks the
motor neuron and causes muscle atrophy due to the lack of innervations. The
cause of the disease is presently unknown. Amyotrophic is derived from the
neurogenic atrophy of the muscle. Lateral sclerosis refers to the hardened spinal cord observed during autopsy. This is due to the proliferation of the glia
cells, astrocytes, and the scarring of the lateral columns. The disease does
not affect sensory neurons or, somewhat surprisingly, the motor neurons
associated with the viscera and glands. The search for the cause and a cure
continues. The investigations are presently focused more in the exploratory
and understanding stage than in the preventative and or curing position.
Summary
The nervous system is a continuous entity that is partitioned, for the sake of
discussion and study, in two parts: the peripheral and central nervous systems. The peripheral nervous system is made up of 12 cranial and 31 pairs of
spinal nerves. The spinal nerves each innervate a particular part of the body
surface. The body surface served by a single spinal nerve is called a
dermatome. The spinal nerves diverge and mix with peripheral nerves so that
the severance of a single spinal nerve causes only a partial loss of sensitivity.
The central nervous system is partitioned in two parts: the spinal cord
and the brain. These two parts function as a unit and are, in fact, structurally a
single continuous system. The spinal cord receives information from the environment via the afferent axons that enter the dorsal horn. Efferent information is sent to muscles and glands via the ventral horn. The division of the spinal nerves in two functional sections is known as the Bell-Magendie law. The
spinal cord both conducts as well as integrates information it receives. The
conductive aspect of the cord is done by the pathways that ascend and descend via the dorsal column, spinothalamic tract, pyramidal tract, lateral
spinothalamic tract, extrapyramidal tract, ventral column, and Lissauer’s
tract. Both the dorsal column and the lateral spinothalamic tract are sensory
in nature and send information to higher nervous centers via the thalamus.
The dorsal column first-order afferents enter the spinal cord and ascend
ipsilaterally to the medulla within the brain stem. The second-order fibers
then cross the midline and ascend via the medial lemniscus. Each hemisphere
of the brain, therefore, processes sensory information from the contralateral
side of the body; the left hemisphere processes information from the right
side of the body and the right hemisphere receives and processes information
from the left side of the body. The third-order fibers leave the thalamus and
project to their final destination in the postcentral gyrus of the cortex. The integrative aspects of the cord are done by the interneurons within the spinal
gray matter (butterfly shaped). Layers 1 through 5 of the interneurons integrate and modulate the information as it passes through the cord on its way
toward or away from the higher centers of the brain.
The two hemispheres of the cortex, separated by the longitudinal fissure
are structurally and functionally connected via a large band of fibers called
the corpus callosum. Each hemisphere is composed of four lobes: frontal,
parietal, occipital, and temporal. Each lobe has been identified with sensory
and motor functions. The motor system is found in the frontal lobes
(precentral gyrus) and the somesthetic sense is in the parietal lobes
(postcentral gyrus). The fissure of Rolando (central fissure) divides the frontal and parietal lobes. The occipital lobes, located posterior to the parietal
lobes, process visual information. The temporal lobes, separated from the parietal and frontal lobes by the Sylvian sulcus,processes auditory information.
This chapter has taken a rather large, complex, and continually growing
body of literature and presented a very simplified story of the chemical and
electrical events that are the basis of neural operation. Because the story is
necessarily brief and incomplete, some liberties were occasionally taken to
ensure, within the author’s capabilities, that the fascinating operation of neurons were clear. As an aid for your understanding, an overview of the cell operation is given next to highlight the main points discussed:
- The neural membrane is
semipermeable. It contains gates or channels through which positive and
negative ions may flow.
- The voltage potential
across the membrane, from inside to outside, is approximately –70 mv when the
neuron is not “active.” The –70 mv within the cell, relative to the outside, is
determined by the K+ ions. The cell, at this point, is at the resting potential.
- The difference between
the –75 mv and the resting potential of –70 mv is due to the leakage of Na+ and
K+ ions into and out of the cell.
- When the leakage is
sufficient to increase the –75 mv to a value of –70 mv, the cell initiates an
active process to maintain the resting state. The active transport of the Na+
and K+ ions—Na+ out and K+ in—by the sodium-potassium pump ensures that the
cell does not become depolarized (go to zero).
- The positive value of
+55 mv occurs at the maximum point of the action potential because of the
sodium influx.
- Chlorine does not enter
the activity of the neuron in terms of the resting potential and action
potential. Calcium also does very little to affect the state of the neuron. The
Ca2+ ions are important, however, when it comes to synaptic transmission.
- The membrane changes
permeability in response to voltage changes across it. In other words, changes
in potential across the membrane opens the Na+ channel to allow Na+ to rush in
because of concentration and electrical gradients (passive transport). The K+
channels are almost immediately closed during the Na+ influx. The brief influx
of Na+ is approximately a millisecond in duration. At the peak of the spike
(+55 mv), the Na+ gates close immediately followed by the reopening of K+
channels.
- The reopening of the K+
channels allows the K+ ions to “escape” to the outside due to high electrical
and concentration gradients. The efflux of the K+ to the outside of the cell
causes the return of the negative internal potential.
- The efflux of the K+ ,
in fact, results in a hyperpolarization of the cell to a value below the –70 mv
resting state.
- The sodium-potassium
pump begins operation following the action potential to prevent the eventual
“running down” of the cell. The tremendous number of ions inside and outside
the cell almost ensures that there can be thousands of spikes before the cell
becomes depleted and all the Na+ and K+ become equal across the cell membrane.
The sodium-potassium pump is not in operation at the point when an action
potential is being actively generated.
- The conductance of the
action potential down the axon is dependent on the size of the axon and whether
or not it is covered with myelin. The large myelinated neurons conduct
at a slower rate the action potential, non-decrementally, at a rapid rate. Small
diameter unmyelinated neurons conduct at a slower rate.
References:
Brodal, A. (1981). Neurological anatomy in relation to
clinical medicine (3rd
ed.). New York: Oxford University Press.
Heimer, L. (1983). The human brain and spinal cord:
Functional neuroanatomy and dissection guide. New York: Springer.
Hodgkin, A. L. (1964). The conduction of the nervous
impulse. Springfield, IL:
Charles C Thomas.
Hodgkin, A. L. (1992). Chance and design: Reminiscences of
science in peace
and war. Cambridge, UK: Cambridge University Press.
Kandel, E. R., Schwartz, J. H., & Jessell, T. M. (1995).
Essentials of neural science and behavior. Norwalk, CT: Appleton & Lange.
Kimelberg, H. K., & Norenberg, M. D. (1989, August).
Astrocytes. Scientific
American, pp. 88-95.
Peters, A., Palay, S. L., & Webster, H. de F. (1991).
The fine structure of the nervous system: Neurons and their supporting cells
(3rd ed.). New York:
Oxford University Press.
Posner, M. I. (Ed.). (1989). Foundations of cognitive
science. Cambridge: MIT
Press.

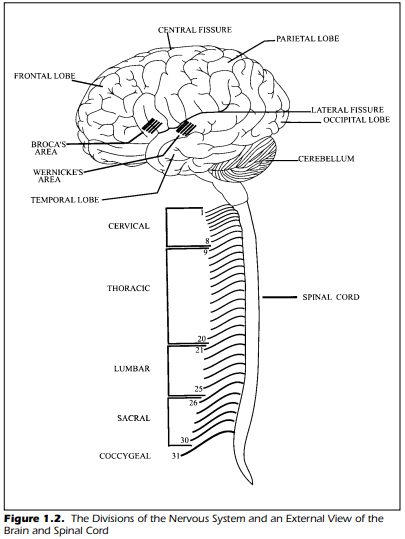





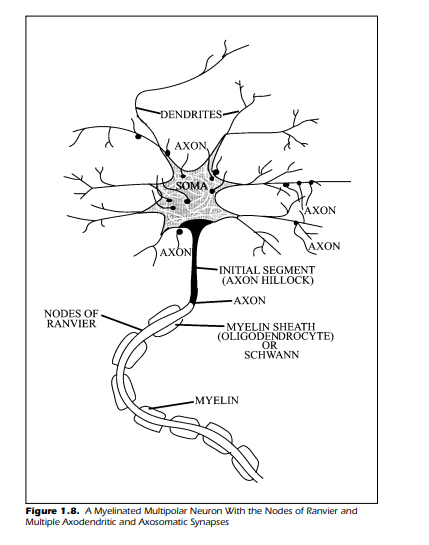








Comments
Post a Comment