CELL COMMUNICATION
Brief table of contents
- Cell Signaling
- Between separate organisms of the same species
- Classification
- In Multicellular Organisms
- In plants
- Cell To Cell Signaling in plants
- Paracrine signaling
- Synaptic signaling
- Synaptic signaling
- Autocrine signaling
- Endocrine signaling
- Signaling through cell-cell contact
- Signaling in plants occurs through plant hormone
- Signaling in plants occurs through Phytochrome
- Phytochrome Signaling Mechanisms
- PLANT PHYTOCHROMES
- Signaling Receptors
- The receptor-ligand interaction can be classified as:
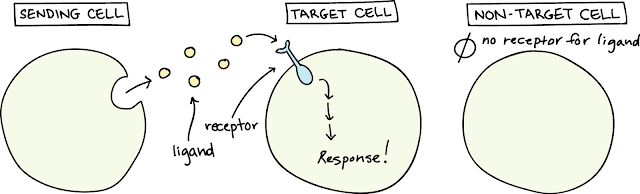
- Overview of Signaling:
- Key components of the signal transduction pathway
What is Signaling?
Cell Signaling
- Synthesis and release of the signaling molecule by the signaling cell ;
- Signal transport to the target cell ;
- Binding of a signal to a specific receptor, leading to its activation ;
- Initiation of signal transduction pathways.
Between separate organisms of the same species
Example:
Classification
- Intracrine signals are produced by the target cell, which remains within the target cell. See More
- Autocrine signals are produced by the target cell, secreted, and act on the target cell itself. through receptors. Sometimes, autocrine cells can target nearby cells if they are the same type as the emitting cell. An example of this is immune cell signals. See More
- Juxtacrine is directed to neighboring (adjacent) cells. These signals are transmitted along cell membranes through protein or lipid components embedded in the membrane and are able to act either on the emitting cell or directly adjacent cells. See More
- Paracrine signals to target cells in the vicinity of the emitting cell. Neurotransmitters are an example. See More
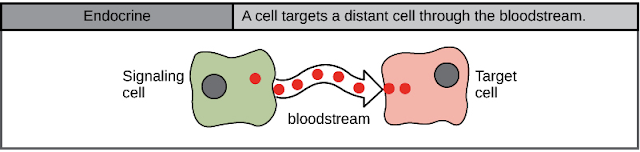
- Endocrine signals target distant cells. Endocrine cells produce hormones that travel through the bloodstream to reach all parts of the body. See More
In Multicellular Organisms
In plants
Cell To Cell Signaling in plants
Paracrine signaling
Synaptic signaling
Synaptic signaling
Autocrine signaling
Endocrine signaling
Signaling through cell-cell contact
Signaling in plants occurs through plant hormone
Plant growth and development are regulated by a structurally unrelated collection of small molecules called plant hormones. During the last 15 years, the number of known plant hormones has grown from five to at least ten. Furthermore, many of the proteins involved in plant hormone signaling pathways have been identified, including receptors for many of the major hormones. Strikingly, the ubiquitin-proteasome pathway plays a central part in most hormone-signaling pathways. In addition, recent studies confirm that hormone signaling is integrated at several levels during plant growth and development.
Because plants have a sessile lifestyle, they must adjust to numerous external stimuli and coordinate their growth and development accordingly. The plant hormones, a group of structurally unrelated small molecules, are central to the integration of diverse environmental cues with a plant’s genetic program. The ‘classical’ phytohormones, identified during the first half of the twentieth century, are auxin, abscisic acid, cytokinin, gibberellin, and ethylene. More recently, several additional compounds have been recognized as hormones, including brassinosteroids, jasmonate, salicylic acid, nitric oxide, and strigolactones (Table)
(Table 1)
Plants also use several peptide hormones to regulate various growth responses, but this class of hormones is beyond our scope here. With the application of genetic approaches, mainly in Arabidopsis thaliana, many aspects of hormone biology have been elucidated. Most hormones are involved in many different processes throughout plant growth and development. This complexity is reflected by the contributions of hormone synthesis, transport, and signaling pathways, as well as by the diversity of interactions among hormones to control growth responses. Genetic screens resulted in the identification of many of the proteins involved in hormone signaling and the analysis of these proteins has contributed significantly to our current models of hormone action. One particularly exciting outcome is the recent identification of receptors for auxin, gibberellin, jasmonate, and abscisic acid (Fig. 1).
{BRI1 is a membrane associated receptor that cycles between the plasma membrane and endosomal compartments. The extracellular leucine-rich repeat domain binds brassinosteroids and transduces the signal through an intracellular kinase domain. GTG1 and GTG2 are GPCR-type G proteins that bind abscisic acid. They have inherent GTPase activity but also interact with the only canonical Ga subunit in Arabidopsis. PYR1/RCAR1 is a soluble ABA receptor that represses PP2C phosphatases in the presence of ABA. The cytokinin receptors CRE1, AHK2, and AHK3 are plasma-membrane associated and perceive cytokinin through their extracellular domains. Cytokinin binding triggers a phosphorylation cascade that is ultimately transmitted to response regulators in the nucleus. Like the cytokinin receptors, the known ethylene receptors are two-component regulators. All five receptors are active in the endoplasmic reticulum and transmit their signal through a common downstream component called CTR1. TIR1 and COI1 are F-box proteins that are integral components of SCF-type E3 ligases and recognize the plant hormones auxin and jasmonic acid respectively. GID1 is a nuclear-localized receptor for gibberellins. Gibberellin binding to GID1 results in the enhanced degradation of DELLA proteins.}
Though far from complete, our improved understanding of hormone perception and signaling has allowed for comparisons between hormones. From there it is clear that some hormones (cytokinins, ethylene, and brassinosteroids) use well-characterized signaling mechanisms. On the other hand, the identification and characterization of the auxin and jasmonate receptors, as well as proteins in gibberellin signaling, have highlighted a novel mechanism for hormone perception in which the ubiquitin-proteasome pathway has a central role. In addition to these advances, the comparison of hormone signaling pathways between evolutionarily tractable members of the plant kingdom has yielded some important insights into the conservation and evolution of hormone signaling pathways. These comparisons have been facilitated by large-scale genome-sequencing projects such as those of Physcomitrella patens (moss), Selaginella (fern), Arabidopsis thaliana, and Oryza sativa (rice). For example, the moss genome (an ancient plant ancestor) encodes proteins that function in auxin, abscisic acid, and cytokinin signaling, whereas the genome of green algae does not, suggesting that these pathways emerged when plants were colonizing land. In contrast, a comparison of the moss genome with more recently diverged plant genomes suggests that signaling mechanisms for gibberellin, ethylene, and the brassinosteroids probably did not evolve until after the evolutionary split of moss and vascular plants. These observations will be expanded as additional hormone signaling components are identified and more genome sequences become available. This is an exciting time in the field of plant hormone biology because our knowledge of hormone biosynthesis, metabolism, transport, perception, signaling and response has grown exponentially over the past few years. As a result, recent reviews have been written for individual hormones covering topics from metabolism and transport to signaling. Here, we review some of the advances in plant hormone signaling. We focus on newly identified hormone receptors and the broad role of regulated protein turnover in plant hormone signaling pathways. We also discuss some of the ways that hormone pathways are integrated during plant growth and development.
Auxin perception by a new class of receptor
Auxin is crucial in regulating plant growth and development from embryogenesis through maturity. As were most hormone signaling proteins identified in plants, the auxin receptors were first found through mutant screens. In this case, the screen was for Arabidopsis seedlings with an altered response to auxin or auxin-transport inhibitors. Many of the auxin-resistant mutants identified in this way are disrupted in components of the Skp1/Cullin/F-box (SCF) ubiquitin ligases (E3) or in proteins that regulate SCF activity.
The E3 ligases are the last enzymes in the ubiquitin-protein conjugation pathway and confer specificity to the pathway. In the case of SCF-type E3 ligases, the F-box protein interacts directly with the substrate and thus determines the substrate specificity of the complex30. SCFs were first implicated in auxin signaling with the identification of an F-box protein called TIR1. Recessive mutations in TIR1 confer auxin resistance, implying that the protein is required for the degradation of negative regulators of auxin response. A key event in the characterization of the auxin-signaling pathway was the discovery that SCF(TIR1) is directly linked to auxin-regulated transcription.
The auxin transcriptional response is controlled by two large families of transcription factors; the auxin/indole-3-acetic acid (Aux/IAA) proteins and the auxin response factors (ARFs) (of which Arabidopsis has 29 and 23 members respectively). ARFs bind the promoters of auxin-responsive genes and either activate or inhibit transcription depending on the type of ARF33. The Aux/IAA proteins bind to the ARFs through shared domains in both proteins called domains III and IV and repress auxin-regulated transcription34. Importantly, the Aux/ IAA proteins are short-lived; their degradation is promoted by auxin and dependent upon TIR1. Many gain-of-function mutations in Aux/ IAA genes have been isolated and in every case the mutations affect residues within a highly conserved region called domain II3. Biochemical studies demonstrated that domain II binds TIR1 and that this binding is enhanced by auxin. Although these results suggested a mechanism for auxin-dependent de-repression of transcription, how auxin promotes the SCFTIR1– Aux/IAA interaction remained unclear. Ultimately, TIR1 itself was shown to bind biologically active auxins directly and specifically9,11. Auxin binding to TIR1 increases the stability of the TIR1–Aux/IAA complex. Structural studies of TIR1 in the presence of auxin and a peptide encompassing domain II revealed how auxin promotes Aux/ IAA degradation. A single hydrophobic pocket on the surface of the leucine-rich repeat domain of TIR1 binds both auxin and the domain II peptide. Auxin binds to residues at the base of this pocket and contributes to binding of the Aux/IAA protein. Domain II of the canonical Aux/IAAs interacts with TIR1 residues directly above auxin, filling the remainder of the pocket. One important implication of the structure is that both TIR1 and the Aux/IAAs appear to contribute to high-affinity binding of auxin. In this sense, it may be more appropriate to call TIR1 and the Aux/IAA protein co-receptors. If true, this also implies that different combinations of F-box protein and substrate may have unique auxin-binding characteristics. Auxin research has a long history and the discovery that TIR1 functions as an auxin receptor was groundbreaking in several respects. The work indicates that F-box proteins, and perhaps other E3 ligases, can function as receptors for small molecules. Indeed, studies have demonstrated that this is probably true (see jasmonate signalling below). Further, the discovery that a small molecule can significantly enhance the interaction between an E3 and its substrate presents a new strategy for the development of drugs that target the ubiquitin–proteasome pathway. Finally, detailed knowledge of auxin receptor function may stimulate the development of new plant growth regulators. Recent results have also shed new light on the mechanism of Aux/ IAA repression. Earlier studies showed that conserved domain I in the Aux/IAA proteins is required for transcriptional repression but the mechanism of repression was unclear. Domain I of most Aux/IAAs contains an ethylene-response-factor-associated amphiphilic repression motif. In 2008, a protein called TOPLESS (TPL) was shown to associate with domain I of the Aux/IAA protein IAA12 and to function as a transcriptional co-repressor. These findings support an updated model in which the Aux/IAA proteins act as repressors of ARFmediated transcription by recruiting TPL or related transcriptional co-repressors to the multi-protein complex. Auxin de-represses transcription by promoting ubiquitination and subsequent degradation of Aux/IAA proteins through the action of SCFTIR1. Without the Aux/ IAA proteins, TPL is no longer associated with promoters of auxinregulated genes (Fig. 2).
Important questions about this model still remain. For example, it is not known whether SCFTIR1 interacts with the Aux/IAA while in a complex with TPL and an ARF, with an ARF alone or perhaps by itself. In addition, each of the relevant proteins is part of a large family (6 TIR1/AFBs, 29 Aux/IAAs, 23 ARFs, 5 TPL/TOPLESS RELATEDs) and the potential specificity of interactions between different family members is just beginning to be explored. If you want to read a complete research paper Click here.
Signaling in plants occurs through Phytochrome
Phytochrome Signaling Mechanisms
INTRODUCTION
PLANT PHYTOCHROMES
The Discovery and Action Modes of Phytochromes
Chromophores and Two Reversible Forms of Phytochromes
Absorption spectra of phytochromes.
Signaling Receptors
The receptor-ligand interaction can be classified as:
- Agonism: This is when the ligand increases the activity of the ligand. Agonism is demonstrated in the absence of any other competing ligand for the same receptor.
- Inverse agonism: when the receptor is constitutively active and the constitutive activity is suppressed or inhibited by the ligand
- Antagonism: In the presence of an agonist ligand, the antagonist molecule prevents the ligand from activating the receptor.
- Partial agonism: this is when the ligand exhibits agonism, but despite increasing the dosage of the ligand, activation of the receptor does not reach a state of full activation.
- Partial inverse agonism: When the receptor is constitutively active, and despite an increase in ligand dosage, the activity of the receptor is decreased but reduced. do not become completely inactive.
- Protein agonism: Protein agonists can act as an agonist or inverse agonist depending on whether the receptor is already inactive (at rest) or already active.
- Biased agonism: when the receptor acts on more than one variant of the next molecule in the transduction chain; and binding to a single agonist favors only one of the possible transduction pathways.
Overview of Signaling:

















Comments
Post a Comment